Prevention of colorectal cancer and dietary management
Introduction
Colorectal cancer (CRC) is among the most commonly diagnosed cancers, with about 1 million new cases and 600,000 deaths worldwide each year (1). Incidence rates vary markedly around the world, with the highest incidence in Australia and New Zealand, Europe and North America, and the lowest in Africa and South-Central Asia (Figure 1) (2). Because of the high incidence of colorectal cancer in Western countries, it is commonly regarded as a Western life-style disease. However, the incidence rates have been increasing in economically transitioning countries, including Eastern European countries, most parts of Asia, and select countries of South America (3).
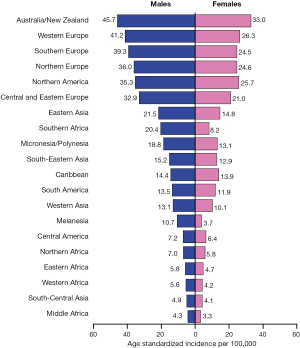
The majority of CRCs are sporadic rather than familial, despite the striking increase in incidence that results from the form of the disease associated with inherited susceptibility (e.g., hereditary non-polyposis colon cancer, HNPCC, and familial adenomatous polyposis, FAP). The role of diet and lifestyle factors has long been suspected and investigated in CRC development, with specific dietary constituents, in addition to excessive caloric intake, weight gain, physical inactivity, smoking, and heavy alcohol intake all thought to result in elevated risk (4,5). The differences in rates by country, and elevated risk among immigrants from a low- to high-risk country (6), support that environmental factors are important in CRC risk. In one study, it was estimated that dietary factors contributed to nearly 50% of all CRC cases diagnosed, while the attributable risk was only about 10% for family history (7). Therefore, a healthy diet and lifestyle are seen as essential in primary prevention of CRC (8), and this review aims to summarize the most up-to-date evidence for these modifiable risk factors. With respect to additional risk modifying interventions, chemoprevention has been a major goal in cancer, and colorectal cancer is seen as amenable to this approach through safe and cost effective agents. We also summarize evidence to date on chemoprevention of CRC.
This review evaluated and summarized scientific evidence on dietary, lifestyle, and chemo-prevention of colorectal cancer. We searched the PubMed database for studies published in English, for key epidemiological studies and animal studies, large case-control and cohort studies, randomized controlled trials (RCTs), and meta-analyses of studies of these different types in humans, with particular focus on studies from the past decade. We summarized the findings in the text and listed key points in Table 1 by level and source of evidence.
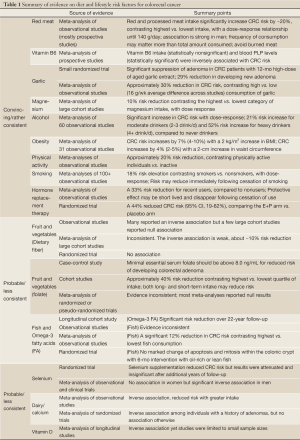
Full Table
Dietary and nutritional components
Red meat
Whether red meat is a culprit in causing CRC has been the subject of scientific debate. Extensive evidence suggests that long-term consumption of red meat or processed meats may increase CRC risk (9-11). A recent meta-analysis of 21 prospective studies showed that consumption of red and processed meats was associated with elevated CRC risk [relative risk, RR=1.22, 95% confidence interval (CI), 1.11-1.34, contrasting the highest versus lowest quartiles of intake], with a linear increase of CRC (RR=1.14, 95% CI, 1.04-1.24) associated with every 100 g/day increase of meat intake until a plateau was encountered at 140 g/day (12). Another meta-analysis of 25 prospective studies that same year found the association was similar by tumor site (colon cancer: RR=1.11, 95% CI, 1.03-1.19, and rectal cancer: RR=1.19, 95% CI, 0.97-1.46) but differed between men (RR=1.21, 95% CI, 1.04-1.42) and women (RR=1.01, 95% CI, 0.87-1.17) (13). Another meta-analysis of both cohort and case-control studies (22 in total) reported that the frequency of red meat consumption rather than total amount of consumed meat is associated with a higher CRC carcinogenesis (14). In addition, the processing method may matter. Very well-cooked meat or meats cooked in direct contact with flames were reported to raise the CRC risk, which may be explained by the carcinogenic heterocyclic amines produced during cooking (15,16). Other plausible biological mechanisms include endogenous formation of nitroso compounds in the gastrointestinal tract by red meat intake, but not by white meat intake (17).
Fruit and vegetables (fiber and folic acid)
Many observational studies have reported an inverse association between dietary fiber and CRC risk, with a relative reduction of up to 40% (18-22), although a few large cohort studies reported small, statistically null associations (23-25). A large pooled analysis of thirteen prospective cohorts suggested that dietary fiber intake was inversely associated with CRC risk in age-adjusted analyses, but no association remained after accounting for other dietary risk factors, including red meat, alcohol, folate, and total milk (26). A recent meta-analysis reported a dose-response analysis indicating that for each 10 g/day total dietary fiber, the relative risk of developing CRC was 0.90 (95% CI, 0.86-0.94) (27). However, two randomized trials found that high dietary fiber did not affect the recurrence of colorectal adenoma (28,29).
Dietary folate or folic acid (from dietary supplements and food fortification) is necessary to synthesize, repair and methylate DNA. It is especially important during periods of rapid cell division and growth such as pregnancy and infancy. Folate is also thought to help prevent changes to DNA that may lead to cancer, and its rule in CRC carcinogenesis has been extensively studied. Humans cannot synthesize folate de novo, and therefore folate has to be supplied through diet to meet their daily requirements, with fresh fruits and vegetables being the major sources. There are growing data and a continuing controversy over the effect of folic acid supplementation on cancer risk. Both the Nurse’s Health Study and secondary analysis from the Wheat Bran Fiber randomized trial reported that high dietary folate was associated with 40% reduced risk of colorectal adenoma (highest vs. lowest quartile) (30,31). The Nurses’ Health Study and Health Professionals Follow-Up Study further suggested that both long- and short-term intakes of total folate were associated with a lower risk of colorectal adenoma (32). With respect to dose, one case-control study based on suspected patients undergoing screening reported that the minimal essential serum folate concentrations should be above 8.0 ng/mL for reducing risk of developing colorectal adenoma (33). One meta-analysis summarized 3 randomized or pseudo-randomized trials and concluded that folate status was inversely related to the risk of developing CRC (34); however, a recently published larger meta-analysis of 15 randomized trials reported that folic acid supplementation has no significant effect on CRC risk (35). A meta-analysis of 3 large randomized trials of folic acid supplementation among patients with an adenoma history also reported no association (36).
Garlic
Garlic is characterized by a high content in organo-sulfur compounds and flavonoids. The allyl sulfur constituents in garlic comprise about 1% of its dry weight and are responsible for its health benefits (37,38). Garlic also contains other constituents such as flavonoids and selenium that are considered to have antioxidant properties and anticarcinogenic activity. Preclinical investigations provide convincing evidence that garlic and related sulfur-containing compounds inhibit carcinogen-induced tumors in various organs (39). One RCT conducted within a small sample of 37 patients with CRC found (I) a significant suppression in both the total size and number of adenomas in CRC patients after 12-mo of high-dose aged garlic extract (40), and (II) a 29% reduction in developing at least one new adenoma (41). A meta-analysis of 4 case control and 3 cohort studies confirmed the inverse association between garlic intake and CRC risk, with an approximate 30% relative reduction in incidence contrasting high vs. low (16 g/wk average difference across studies) consumption of garlic (42).
Fish and Omega-3 fatty acids
Long-chain n-3 fatty acids have been suggested to play a protective role in colorectal cancer development in laboratory and animal studies, with the mechanism of action conjectured to be inhibition of the cyclooxygenase-2 (COX-2) enzyme and the production of arachidonic acid (n-6) derived eicosanoids (40-42). A long-term prospective study of U.S. men reported a significantly reduced CRC risk contrasting the highest versus lowest quartiles of n-3 fatty acids (odds ratio, OR=0.74, 95% CI, 0.57-0.95) (43). Fish is the main dietary source of the long-chain n-3 fatty acids. Some observational studies found an inverse association between fish consumption and CRC risk (10,43-45), while others did not (46-48). A recent meta-analysis pooled data from 22 cohort and 19 case-control studies, and reported a 12% (OR=0.88, 95% CI, 0.80-0.95) reduction in CRC risk contrasting highest vs. lowest fish consumption (49). However, a multi-center, randomized controlled trial investigated the effect of 6-month intervention with oil-rich or lean fish on apoptosis and mitosis within the colonic crypt, and found no marked change in these endpoints (50).
Vitamin B6
Vitamin B6 is widely distributed in foods, with good sources including meats, whole grain products, vegetables, nuts and bananas. Vitamin B6 is involved in almost 100 enzymatic reactions, among which one function involves transferring 1-carbon groups for DNA synthesis and methylation (51). Therefore, vitamin B6 deficiency may increase CRC risk through aberrations in DNA synthesis, repair, and methylation. Vitamin B may also suppress colorectal carcinogenesis by reducing cell proliferation, angiogenesis, oxidative stress, inflammation, and nitric oxide synthesis (52,53). A meta-analysis summarized evidence from prospective studies, with 9 studies on vitamin B6 intake and 4 studies on blood pyridoxal 5'-phosphate (PLP, the principal active coenzyme form of vitamin B6) levels, in relation to CRC risk. The pooled RRs of CRC contrasting highest vs. lowest category of vitamin B6 intake and blood PLP levels were 0.90 (95% CI, 0.75-1.07) and 0.52 (95% CI, 0.38-0.71), respectively (54).
Dairy, calcium and vitamin D
In vitro and in vivo studies have suggested that high dietary intake of calcium and vitamin D may reduce CRC risk by a variety of mechanisms, such as reducing epithelial cell exposure to toxicity, inhibiting proliferation of intestinal mucosa and epithelial cells via the intracellular action of calcium (55); Vitamin D’s protective effect against colorectal neoplasia is by reducing epithelial cell proliferation (56). There have been numerous epidemiological studies on calcium and CRC risk, as well as studies on vitamin D and CRC risk. However, there remains a lack of clarity of the effects on CRC of both these dietary components, due to inconsistent findings among numerous epidemiological studies and multiple pooled analyses. Overall, results from observational studies found calcium intake to be not associated with a substantially lower CRC risk (57,58) particularly for the more reliable large prospective studies (57). A meta-analysis of summarizing a large number of observational studies mostly supported an inverse association for both calcium and milk/dairy products (a rich source of both calcium and vitamin D) (59,60). The evidence from randomized trials only shows a protective effect of calcium on adenoma recurrence among individuals with a history of adenomas (RR=0.80, 95% CI, 0.69-0.94) for those receiving calcium 1,200 to 2,000 mg/d, but no effect in general populations (RR=0.62, 95% CI, 0.11-3.40) (59). It should be noted that the statistical power in the two clinical trials conducted in general populations is limited (59).
In 1980, Garland hypothesized that lower levels of vitamin D resulting from weaker UV-B radiation at higher latitudes may account for the striking geographical pattern of CRC mortality (60). The evidence from epidemiologic studies are limited to small sample sizes, yet in general found vitamin D inversely associated with CRC risk (57,61-64). Meta-analyses of longitudinal studies reported a 50% lower CRC risk associated with a serum 25(OH)D level ≥33 ng/mL, compared to ≤12 ng/mL (65), or an OR of 0.57 (95% CI, 0.43-0.76) for an increase of 25(OH)D by 20 ng/mL (66).
Selenium
As an essential trace element involved in different physiological functions in the human body, selenium has received increasing attention as a possible cancer prevention substance, through several possible mechanisms for the potential anticarcinogenic effects including apoptosis (67), protection from oxidative DNA damage (68), and increased immune function (69). However, the epidemiologic evidence on associations between selenium and CRC risk has been mixed, inconclusive and limited to small studies. Many observational studies support a protective effect of selenium on CRC risk (70-72) but not consistently (73,74). A meta-analysis of 12 observational studies and 2 clinical trials reported no association between selenium and CRC risk in women, but an inverse association in men (OR=0.68, 95% CI, 0.57-0.82) (75). There was a large randomized trial on selenium supplementation for skin cancer prevention, and the secondary analysis showed a 58% reduction (95% CI, 0.18-0.95) in CRC incidence among participants randomly assigned to take selenium (200 µg daily) (76), although the results were attenuated and no longer statistically significant after additional years of follow-up (77). A pooled analysis of 3 randomized trials on various nutritional interventions for CRC prevention reported a pooled OR of 0.66 (95% CI, 0.50-0.87), contrasting participants of highest vs. lowest blood selenium values (78). Yet, none of these trials were designed to test selenium as an intervention, and all participants were at high adenoma risk after recent colonoscopic adenoma resection (79).
Magnesium
Magnesium is abundant in many foods, and particularly rich in spices, nuts, cereals, coffee, cocoa, tea and green-leafy vegetables. Magnesium is involved in a wide variety of biochemical reactions that modulate key cell functions, and has a crucial role in genomic stability and DNA synthesis (80). Epidemiologic studies suggested that magnesium may be associated with a decreased CRC risk but the findings are inconsistent. A meta-analysis of 8 prospective studies containing 338,979 participants and 8,000 CRC cases reported a summary RR of 0.89 (95% CI, 0.79-1.00) for the highest vs. lowest category of magnesium intake, with evidence of a dose-response (81).
Dietary pattern, obesity and related mechanisms
Dietary pattern summarizes the total diet or the key dietary components including food items, food groups, and nutrients (82), and may provide additional insights with the combined effects of many food components. Summarized epidemiologic evidence suggest that healthier pattern consisting of greater intakes of fruits and vegetables, and lower intakes of red and processed meat, appeared protective against colorectal adenoma and cancer incidence, while a less healthy pattern characterized by higher intakes of red and processed meat, as well as potatoes and refined carbohydrates, may increase risk (83). There has also been great interest in total dietary consumption and its consequences, specifically obesity, in relation to colorectal and other cancers (84). Because these risks are modifiable, these pathways are seen as key in cancer control efforts (85). Colon cancer is among those specifically identified as linked to obesity; for example, two large prospective cohort studies have demonstrated that being obese [body mass index (BMI) >30 kg/m2] confers a 1.5-fold greater risk of developing colon cancer relative to individuals of normal weight (BMI 18.5-24.9 kg/m2) (86,87). With respect to mortality, the Cancer Prevention Study II, which prospectively followed more than 900,000 US adults, similarly found a 1.5- to 1.8-fold increased risk of colon cancer related death for obese individuals, reflecting the increased incidence of and perhaps the increased mortality from colon cancer once diagnosed (88). A meta-analysis of 31 observational studies reported that for a 2 kg/m2 increase in BMI, the CRC risk increased by 7% (95% CI, 4-10%), and for a 2-cm increase in waist circumference, the risk increased by 4% (95% CI, 2-5%) (89). Another meta-analysis showed that the inverse association between BMI and CRC risk was stronger for colon than rectal cancer, and for men than women (90). Some recent research probing obesity pathways concern the issue of ‘secondary prevention’ after diagnosis and treatment of early stage disease. Following earlier studies by Meyerhardt et al. (91) and Dignam et al. (92), which found excess risk of both cancer recurrence and overall mortality among obese patients utilizing large participant cohorts from multicenter clinical trials for stage II and III colon cancer, subsequent studies turned to elucidating the role of dietary constituents among colon cancer patients. The first of these studies examined the ‘Western’ diet pattern characterized by relatively high red and processed meats, refined grain, and sugar content, and indicated that those with higher consumption of these components experienced significantly elevated recurrence and mortality risk (93). A more recent study more closely examined the Western diet and the role of dietary glycemic measures (94). This study found that high carbohydrate intake and glycemic load significantly increased recurrence and mortality. These effects were present among all body types but strongest among those who were overweight or obese. In relation to CRC risk, the Western dietary pattern has previously been reported to be associated with an elevated CRC incidence (95). However, the Women’s Health Initiative Dietary Modification Trial, a large randomized trial conducted in postmenopausal women, suggested that a low-fat dietary pattern intervention did not reduce CRC risk during 8 years of follow-up (96).
This work relates to a large body of research focused on potential mechanisms by which obesity leads to higher colon cancer incidence. One prominent theme involves metabolic and insulin related pathways (97-104). Insulin, insulin-like growth factors (IGF), and IGF binding proteins have all been causatively implicated for colon cancer via mitogenic effects on the colonic mucosa and other mechanisms. In a prospective nested case-control study within the Physicians’ Health Study, Ma et al. (101) found that men in the highest quintile for IGF-1 had a 2.5 increased risk of colorectal cancer compared to the lowest quintile. Among women, an analysis of the Nurse’s Health Study cohort found a comparable >2-fold for those in the highest quartile of IGF-1 compared with those in the lowest (102). That study, as well as investigations by Kaaks et al. (103) and a more recent study by Ma et al. (104) also observed that colorectal cancer risk increased with increasing levels of C-peptide (a marker of insulin production). Interestingly, reduction in IGF exposure via increased presence of its binding protein was found to significantly reduce colon cancer mortality among affected individuals in a large cohort study (105). This particular pathway represents but one of numerous putative links linking cancer and obesity (84).
Obviously, a critical component of obesity is physical activity. The association between physical activity and CRC risk is well established, with the majority of relevant studies finding physically active individuals benefit from substantial risk reduction (approximately 20%) comparing to the sedentary ones, confirmed by two meta-analyses (106,107). The evidence is strong and consistent for both proximal colon and distal colon cancers with a similar magnitude (107). The main challenge is disentangling the direct effects of physical activity from strongly related factors such as obesity and comorbidities that may influence outcomes. Randomized intervention trials have been completed with positive findings, and others are underway; these studies have been mostly focused on colorectal cancer survivors (clinical event reduction) or biomarkers (prevention studies) for practical reasons (108-110).
Other common lifestyle exposures
Alcohol
Alcohol consumption is one of the most important known causes of human cancer, possibly through genotoxic effect of acetaldehyde (111) and interference of folate absorption by alcohol (112). The most recent meta-analysis pooled data from 27 cohort and 34 case-control studies, and reported significantly increased CRC risk with a dose-response relationship (113): comparing to never drinkers, moderate drinkers who consume 2-3 drinks per day have 21% increased CRC risk and heavy drinkers who have 4 or more drinks per day have 52% increased CRC risk. These results are consistent with other pooled analyses, with increased risk observed for both colon and rectal cancers (114-116).
Smoking
Cigarette smoking has been shown to cause many nonpulmonary cancers with no direct tobacco-related carcinogens, yet the association between smoking and CRC remains controversial. A meta-analysis of 106 observational studies estimated that cigarette smokers are 1.18 times (95% CI, 1.11-1.25 times) more likely to develop CRC compared to those who never smoke, with evidence of a dose-response (117). Another meta-analysis of 36 studies reported that duration and age of initiation were also significantly associated with CRC incidence (118). A recent study reported that the excess CRC risk due to smoking decreased immediately after quitting for proximal colon and rectal cancer, but not until about 20 years post-quitting for distal colon cancer (119).
Hormone replacement therapy (HRT)
Estrogen/progestin replacement therapy is prescribed to control postmenopausal symptom or to prevent hormone deficiency-related diseases such as osteoporosis. Existing evidence shows that HRT is associated with lower CRC risk, yet the mechanism remained unclear. Case control studies have shown significant reduction in CRC risk among ever users (120,121). Large cohort studies suggested a modest risk reduction among ever users (122), but there was a lack of dose-response relationship (123). Also, the association was found stronger among women aged 65+, with a body mass index <30 and who regularly use aspirin or ibuprofen (124). A meta-analysis concluded that recent HRT users had a 33% reduction in CRC risk (pooled RR=0.67, 95% CI, 0.59-0.77) but no association was observed with ever use of HRT and duration of use did not matter (125). Other studies also supported that the protective effect of HRT is short-lived and disappears following cessation of use (124). The Women’s Health Initiative trial found that women who took estrogen plus progestin had 44% reduced risk (95% CI, 19-62%) of colorectal cancer compared to women who took placebo but colorectal cancers in the estrogen plus progestin group were at a more advanced stage (126). The Women’s Health Initiative trial later reported that there was no significant difference in colorectal cancer incidence between the estrogen-only and placebo arms (127).
Chemoprevention of colorectal cancer
Prevention of colorectal cancer and its precursor conditions via some substance or compound, or chemoprevention, offers great potential if the intervention is safe and cost-effective. The main focus of these has not been nutritional, but rather medicinal agents with reasonably safe adverse event profiles. For more than two decades, accumulating evidence from observational studies (128-133) and large randomized trials (134-137) suggests that aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) protect against the development of colonic adenomas and CRC, and reduces disease recurrence (136-139). Meta-analysis of 17 case-control studies showed an inverse association between regular use of aspirin and reduced CRC risk (pooled OR=0.62, 95% CI, 0.58-0.67), while the association was weaker in cohort studies presumably due to substantial variation between studies in measuring aspirin exposure (140). Evidence from randomized trials has not been consistent. Two trials conducted among patients with a history of CRC or adenomas showed that low-dose aspirin has a chemopreventive effect on new adenomas (136,137). Yet among healthy individuals, two earlier large trials of low-dose aspirin (the Physicians’ Health Study and the Women’s Health Study) showed no effect on CRC incidence during 10-year follow-up, possibly due to an insufficient dose (134,141,142). Recent long-term follow-up of four aspirin trials (Primary: Thrombosis Prevention Trial, British Doctors Aspirin Trial; Secondary: Swedish Aspirin Low Dose Trial, UK-TIA Aspirin Trial) showed a reduction of 50% in CRC risk after five or more years of regular consumption with a dosage of at least 75 mg daily (75-300 mg) (143). Aspirin has emerged as the most likely NSAID for use in chemoprevention because of its known cardiovascular benefit and available safety and efficacy data (144). Other traditional NSAIDs, particularly selective COX-2 inhibitors such as celecoxib, have been shown to cause regression of adenomas in familial adenomatous polyposis, and are now given to patients at high risk of CRC (138,139), and yet cannot be routinely recommended concerning potential cardiovascular events. The Colorectal Adenoma/carcinoma Prevention Programme (CAPP) was launched in 1990, with CAPP1 investigating familial adenomatous polyposis in 200 young adults and CAPP2 being the first large-scale genetically targeted chemoprevention trial in 1,000 adults with HNPCC (also known as Lynch syndrome). The CAPP2 randomized trial reported that 600 mg aspirin per day for a mean of 25 months substantially reduced CRC incidence among carriers of hereditary CRC (145). However, the minimum dose of aspirin to achieve the protective effect is still uncertain and will be the objective of a new CAPP trial in Lynch Syndrome. The underlying mechanism of NSAIDs inhibiting carcinogenesis remains inconclusive, with proposed explanations as increased apoptosis and impairment of tumor cell growth by inhibition of cyclooxygenase-2 (COX-2) (146).
The side-effects of NSAIDs are well documented and mainly attributed to inhibition of COX activity. The most frequently reported serious adverse events associated with aspirin use are related to gastrointestinal bleeding, even with low-dose aspirin (147). Comparing to other NSAIDs, aspirin has lower risk of occlusive cardiovascular events, yet the dose-dependent risk of bleeding complications with aspirin intake may limit its potential for primary prevention of CRC (148). In additional to optimal dose, optimum treatment duration and age of initiation also remained uncertain (144). Concerning risk and benefit, the United States Preventive Services Task Force recommended against the routine use of aspirin for CRC prevention in 2007 (149). However, the 2011 pooled analysis with 8 randomized controlled trials showed that daily aspirin for 5-10 years reduced 5-year cancer mortality by 34% (P=0.03) and a 20-year cancer mortality by 20% (P<0.001) (150). Considering the bleeding complications are not life-threatening, the risk-benefit of aspirin use in CRC prevention requires a formal re-evaluation. As toxicity of aspirin largely depends on dosage, the minimal effective dose required for CRC prevention is critical (151).
Conclusions
It has been estimated that dietary factors account for nearly half of all colorectal cancer cases, and therefore diet and lifestyle are key intervention points in primary prevention. This review summarized and updated the relevant epidemiologic evidence for dietary constituents and other modifiable factors, some of which are likely correlated with diet behavior. There is convincing, or moderately convincing and rather consistent, evidence that intakes of garlic, vitamin B6 and magnesium, maintaining a healthy weight and waist via both diet and exercise, and avoiding or reducing red meat, alcohol, and smoking, may significantly protect against developing colorectal cancer. There is high quality yet less consistent evidence for the following dietary components: fruit and vegetable intake (fiber and folate), fish and Omega-3 fatty acids, selenium, dairy, calcium and vitamin D. For high risk populations for whom dietary and lifestyle interventions may not be sufficient for primary prevention, aspirin and other chemoprevention agents may be considered, although the minimal effective dose remains unclear. Ongoing studies may shed light on additional safe interventions that can reduce colorectal cancer and will likely have other health benefits.
Acknowledgements
This work was supported by Public Health Service grant NCI P30-CA-14599 from the National Cancer Institute, United States National Institutes of Health.
Disclosure: The authors declare no conflict of interest.
References
- Weitz J, Koch M, Debus J, et al. Colorectal cancer. Lancet 2005;365:153-65. [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev 2009;18:1688-94. [PubMed]
- Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst 1981;66:1191-308. [PubMed]
- Giovannucci E, Stampfer MJ, Colditz G, et al. Relationship of diet to risk of colorectal adenoma in men. J Natl Cancer Inst 1992;84:91-8. [PubMed]
- Flood DM, Weiss NS, Cook LS, et al. Colorectal cancer incidence in Asian migrants to the United States and their descendants. Cancer Causes Control 2000;11:403-11. [PubMed]
- Kune GA, Bannerman S, Watson LF. Attributable risk for diet, alcohol, and family history in the Melbourne Colorectal Cancer Study. Nutr Cancer 1992;18:231-5. [PubMed]
- Shike M. Diet and lifestyle in the prevention of colorectal cancer: an overview. Am J Med 1999;106:11S-15S; discussion 50S-51S.
- Chao A, Thun MJ, Connell CJ, et al. Meat consumption and risk of colorectal cancer. JAMA 2005;293:172-82. [PubMed]
- Norat T, Bingham S, Ferrari P, et al. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. J Natl Cancer Inst 2005;97:906-16. [PubMed]
- Cross AJ, Ferrucci LM, Risch A, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res 2010;70:2406-14. [PubMed]
- Chan DS, Lau R, Aune D, et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One 2011;6:e20456. [PubMed]
- Alexander DD, Weed DL, Cushing CA, et al. Meta-analysis of prospective studies of red meat consumption and colorectal cancer. Eur J Cancer Prev 2011;20:293-307. [PubMed]
- Smolińska K, Paluszkiewicz P. Risk of colorectal cancer in relation to frequency and total amount of red meat consumption. Systematic review and meta-analysis. Arch Med Sci 2010;6:605-10. [PubMed]
- Sinha R, Chow WH, Kulldorff M, et al. Well-done, grilled red meat increases the risk of colorectal adenomas. Cancer Res 1999;59:4320-4. [PubMed]
- Ferrucci LM, Sinha R, Huang WY, et al. Meat consumption and the risk of incident distal colon and rectal adenoma. Br J Cancer 2012;106:608-16. [PubMed]
- Bingham SA, Hughes R, Cross AJ. Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. J Nutr 2002;132:3522S-3525S. [PubMed]
- Levi F, Pasche C, Lucchini F, et al. Dietary fibre and the risk of colorectal cancer. Eur J Cancer 2001;37:2091-6. [PubMed]
- Bingham SA, Day NE, Luben R, et al. European Prospective Investigation into Cancer and Nutrition. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet 2003;361:1496-501. [PubMed]
- Terry P, Giovannucci E, Michels KB, et al. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst 2001;93:525-33. [PubMed]
- Peters U, Sinha R, Chatterjee N, et al. Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Dietary fibre and colorectal adenoma in a colorectal cancer early detection programme. Lancet 2003;361:1491-5. [PubMed]
- Dahm CC, Keogh RH, Spencer EA, et al. Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst 2010;102:614-26. [PubMed]
- Mai V, Flood A, Peters U, et al. Dietary fibre and risk of colorectal cancer in the Breast Cancer Detection Demonstration Project (BCDDP) follow-up cohort. Int J Epidemiol 2003;32:234-9. [PubMed]
- Schatzkin A, Mouw T, Park Y, et al. Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and Health Study. Am J Clin Nutr 2007;85:1353-60. [PubMed]
- Fuchs CS, Giovannucci EL, Colditz GA, et al. Dietary fiber and the risk of colorectal cancer and adenoma in women. N Engl J Med 1999;340:169-76. [PubMed]
- Park Y, Hunter DJ, Spiegelman D, et al. Dietary fiber intake and risk of colorectal cancer: a pooled analysis of prospective cohort studies. JAMA 2005;294:2849-57. [PubMed]
- Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 2011;343:d6617. [PubMed]
- Alberts DS, Martínez ME, Roe DJ, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians’ Network. N Engl J Med 2000;342:1156-62. [PubMed]
- Schatzkin A, Lanza E, Corle D, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N Engl J Med 2000;342:1149-55. [PubMed]
- Giovannucci E, Stampfer MJ, Colditz GA, et al. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst 1993;85:875-84. [PubMed]
- Martínez ME, Henning SM, Alberts DS. Folate and colorectal neoplasia: relation between plasma and dietary markers of folate and adenoma recurrence. Am J Clin Nutr 2004;79:691-7. [PubMed]
- Lee JE, Willett WC, Fuchs CS, et al. Folate intake and risk of colorectal cancer and adenoma: modification by time. Am J Clin Nutr 2011;93:817-25. [PubMed]
- Fujimori S, Gudis K, Takahashi Y, et al. Determination of the minimal essential serum folate concentration for reduced risk of colorectal adenoma. Clin Nutr 2011;30:653-8. [PubMed]
- Fife J, Raniga S, Hider PN, et al. Folic acid supplementation and colorectal cancer risk: a meta-analysis. Colorectal Dis 2011;13:132-7. [PubMed]
- Qin X, Cui Y, Shen L, et al. Folic acid supplementation and cancer risk: A meta-analysis of randomized controlled trials. Int J Cancer 2013; [PubMed]
- Figueiredo JC, Mott LA, Giovannucci E, et al. Folic acid and prevention of colorectal adenomas: a combined analysis of randomized clinical trials. Int J Cancer 2011;129:192-203. [PubMed]
- Ngo SN, Williams DB, Cobiac L, et al. Does garlic reduce risk of colorectal cancer? A systematic review. J Nutr 2007;137:2264-9. [PubMed]
- El-Bayoumy K, Sinha R, Pinto JT, et al. Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds. J Nutr 2006;136:864S-869S. [PubMed]
- Knowles LM, Milner JA. Possible mechanism by which allyl sulfides suppress neoplastic cell proliferation. J Nutr 2001;131:1061S-6S. [PubMed]
- Tanaka S, Haruma K, Yoshihara M, et al. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. J Nutr 2006;136:821S-826S. [PubMed]
- Tanaka S, Haruma K, Kunihiro M, et al. Effects of aged garlic extract (AGE) on colorectal adenomas: a double-blinded study. Hiroshima J Med Sci 2004;53:39-45. [PubMed]
- Fleischauer AT, Poole C, Arab L. Garlic consumption and cancer prevention: meta-analyses of colorectal and stomach cancers. Am J Clin Nutr 2000;72:1047-52. [PubMed]
- Hall MN, Chavarro JE, Lee IM, et al. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev 2008;17:1136-43. [PubMed]
- Jedrychowski W, Maugeri U, Pac A, et al. Protective effect of fish consumption on colorectal cancer risk. Hospital-based case-control study in Eastern Europe. Ann Nutr Metab 2008;53:295-302. [PubMed]
- Kato I, Akhmedkhanov A, Koenig K, et al. Prospective study of diet and female colorectal cancer: the New York University Women’s Health Study. Nutr Cancer 1997;28:276-81. [PubMed]
- Sugawara Y, Kuriyama S, Kakizaki M, et al. Fish consumption and the risk of colorectal cancer: the Ohsaki Cohort Study. Br J Cancer 2009;101:849-54. [PubMed]
- Kobayashi M, Tsubono Y, Otani T, et al. Fish, long-chain n-3 polyunsaturated fatty acids, and risk of colorectal cancer in middle-aged Japanese: the JPHC study. Nutr Cancer 2004;49:32-40. [PubMed]
- English DR, MacInnis RJ, Hodge AM, et al. Red meat, chicken, and fish consumption and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 2004;13:1509-14. [PubMed]
- Wu S, Feng B, Li K, et al. Fish consumption and colorectal cancer risk in humans: a systematic review and meta-analysis. Am J Med 2012;125:551-9.e5.
- Pot GK, Majsak-Newman G, Geelen A, et al. Fish consumption and markers of colorectal cancer risk: a multicenter randomized controlled trial. Am J Clin Nutr 2009;90:354-61. [PubMed]
- Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging 2002;6:39-42. [PubMed]
- Matsubara K, Komatsu S, Oka T, et al. Vitamin B6-mediated suppression of colon tumorigenesis, cell proliferation, and angiogenesis J Nutr Biochem 2003;14:246-50. [PubMed]
- Shen J, Lai CQ, Mattei J, et al. Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: the Boston Puerto Rican Health Study. Am J Clin Nutr 2010;91:337-42. [PubMed]
- Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA 2010;303:1077-83. [PubMed]
- Lipkin M, Newmark H. Effect of added dietary calcium on colonic epithelial-cell proliferation in subjects at high risk for familial colonic cancer. N Engl J Med 1985;313:1381-4. [PubMed]
- Shabahang M, Buras RR, Davoodi F, et al. Growth inhibition of HT-29 human colon cancer cells by analogues of 1,25-dihydroxyvitamin D3. Cancer Res 1994;54:4057-64. [PubMed]
- Martínez ME, Willett WC. Calcium, vitamin D, and colorectal cancer: a review of the epidemiologic evidence. Cancer Epidemiol Biomarkers Prev 1998;7:163-8. [PubMed]
- Bergsma-Kadijk JA, van’t Veer P, Kampman E, et al. Calcium does not protect against colorectal neoplasia. Epidemiology 1996;7:590-7. [PubMed]
- Carroll C, Cooper K, Papaioannou D, et al. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther 2010;32:789-803. [PubMed]
- Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol 1980;9:227-31. [PubMed]
- Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006;354:684-96. [PubMed]
- Garland CF, Comstock GW, Garland FC, et al. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet 1989;2:1176-8. [PubMed]
- Braun MM, Helzlsouer KJ, Hollis BW, et al. Colon cancer and serum vitamin D metabolite levels 10-17 years prior to diagnosis. Am J Epidemiol 1995;142:608-11. [PubMed]
- Feskanich D, Ma J, Fuchs CS, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 2004;13:1502-8. [PubMed]
- Gorham ED, Garland CF, Garland FC, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med 2007;32:210-6. [PubMed]
- Yin L, Grandi N, Raum E, et al. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther 2009;30:113-25. [PubMed]
- Sanmartín C, Plano D, Sharma AK, et al. Selenium compounds, apoptosis and other types of cell death: an overview for cancer therapy. Int J Mol Sci 2012;13:9649-72. [PubMed]
- Ramoutar RR, Brumaghim JL. Effects of inorganic selenium compounds on oxidative DNA damage. J Inorg Biochem 2007;101:1028-35. [PubMed]
- Broome CS, McArdle F, Kyle JA, et al. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr 2004;80:154-62. [PubMed]
- Connelly-Frost A, Poole C, Satia JA, et al. Selenium, folate, and colon cancer. Nutr Cancer 2009;61:165-78. [PubMed]
- Fernández-Bañares F, Cabré E, Esteve M, et al. Serum selenium and risk of large size colorectal adenomas in a geographical area with a low selenium status. Am J Gastroenterol 2002;97:2103-8. [PubMed]
- Clark LC, Hixson LJ, Combs GF Jr, et al. Plasma selenium concentration predicts the prevalence of colorectal adenomatous polyps. Cancer Epidemiol Biomarkers Prev 1993;2:41-6. [PubMed]
- Garland M, Morris JS, Stampfer MJ, et al. Prospective study of toenail selenium levels and cancer among women. J Natl Cancer Inst 1995;87:497-505. [PubMed]
- van den Brandt PA, Goldbohm RA, van’t Veer P, et al. A prospective cohort study on toenail selenium levels and risk of gastrointestinal cancer. J Natl Cancer Inst 1993;85:224-9. [PubMed]
- Takata Y, Kristal AR, King IB, et al. Serum selenium, genetic variation in selenoenzymes, and risk of colorectal cancer: primary analysis from the Women’s Health Initiative Observational Study and meta-analysis. Cancer Epidemiol Biomarkers Prev 2011;20:1822-30. [PubMed]
- Clark LC, Combs GF Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 1996;276:1957-63. [PubMed]
- Duffield-Lillico AJ, Reid ME, Turnbull BW, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev 2002;11:630-9. [PubMed]
- Jacobs ET, Jiang R, Alberts DS, et al. Selenium and colorectal adenoma: results of a pooled analysis. J Natl Cancer Inst 2004;96:1669-75. [PubMed]
- Duffield-Lillico AJ, Shureiqi I, Lippman SM. Can selenium prevent colorectal cancer? A signpost from epidemiology. J Natl Cancer Inst 2004;96:1645-7. [PubMed]
- Hartwig A. Role of magnesium in genomic stability. Mutat Res 2001;475:113-21. [PubMed]
- Chen GC, Pang Z, Liu QF. Magnesium intake and risk of colorectal cancer: a meta-analysis of prospective studies. Eur J Clin Nutr 2012;66:1182-6. [PubMed]
- Randi G, Edefonti V, Ferraroni M, et al. Dietary patterns and the risk of colorectal cancer and adenomas. Nutr Rev 2010;68:389-408. [PubMed]
- Miller PE, Lesko SM, Muscat JE, et al. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer 2010;62:413-24. [PubMed]
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004;4:579-91. [PubMed]
- Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc 2008;67:253-6. [PubMed]
- Martínez ME, Giovannucci E, Spiegelman D, et al. Leisure-time physical activity, body size, and colon cancer in women. Nurses’ Health Study Research Group. J Natl Cancer Inst 1997;89:948-55. [PubMed]
- Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 1995;122:327-34. [PubMed]
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38. [PubMed]
- Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 2007;16:2533-47. [PubMed]
- Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev 2010;11:19-30. [PubMed]
- Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer 2003;98:484-95. [PubMed]
- Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst 2006;98:1647-54. [PubMed]
- Meyerhardt JA, Sato K, Niedzwiecki D, et al. Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Natl Cancer Inst 2012;104:1702-11. [PubMed]
- Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA 2007;298:754-64. [PubMed]
- Fung T, Hu FB, Fuchs C, et al. Major dietary patterns and the risk of colorectal cancer in women. Arch Intern Med 2003;163:309-14. [PubMed]
- Beresford SA, Johnson KC, Ritenbaugh C, et al. Low-fat dietary pattern and risk of colorectal cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:643-54. [PubMed]
- Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc 2001;60:91-106. [PubMed]
- Giovannucci E, Pollak MN, Platz EA, et al. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev 2000;9:345-9. [PubMed]
- Manousos O, Souglakos J, Bosetti C, et al. IGF-I and IGF-II in relation to colorectal cancer. Int J Cancer 1999;83:15-7. [PubMed]
- Schoen RE, Tangen CM, Kuller LH, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst 1999;91:1147-54. [PubMed]
- Ma J, Pollak MN, Giovannucci E, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst 1999;91:620-5. [PubMed]
- Wei EK, Ma J, Pollak MN, et al. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 2005;14:850-5. [PubMed]
- Kaaks R, Toniolo P, Akhmedkhanov A, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst 2000;92:1592-600. [PubMed]
- Ma J, Giovannucci E, Pollak M, et al. A prospective study of plasma C-peptide and colorectal cancer risk in men. J Natl Cancer Inst 2004;96:546-53. [PubMed]
- Haydon AM, Macinnis RJ, English DR, et al. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut 2006;55:62-7. [PubMed]
- Wolin KY, Yan Y, Colditz GA, et al. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer 2009;100:611-6. [PubMed]
- Boyle T, Keegel T, Bull F, et al. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst 2012;104:1548-61. [PubMed]
- Courneya KS, Booth CM, Gill S, et al. The Colon Health and Life-Long Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol 2008;15:279-85. [PubMed]
- Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol 2006;24:3535-41. [PubMed]
- Winzer BM, Whiteman DC, Reeves MM, et al. Physical activity and cancer prevention: a systematic review of clinical trials. Cancer Causes Control 2011;22:811-26. [PubMed]
- Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol 2006;7:149-56. [PubMed]
- Giovannucci E, Rimm EB, Ascherio A, et al. Alcohol, low-methionine--low-folate diets, and risk of colon cancer in men. J Natl Cancer Inst 1995;87:265-73. [PubMed]
- Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol 2011;22:1958-72. [PubMed]
- Mizoue T, Inoue M, Wakai K, et al. Research Group for Development and Evaluation of Cancer Prevention Strategies in Japan. Alcohol drinking and colorectal cancer in Japanese: a pooled analysis of results from five cohort studies. Am J Epidemiol 2008;167:1397-406. [PubMed]
- Moskal A, Norat T, Ferrari P, et al. Alcohol intake and colorectal cancer risk: a dose-response meta-analysis of published cohort studies. Int J Cancer 2007;120:664-71. [PubMed]
- Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med 2004;140:603-13. [PubMed]
- Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300:2765-78. [PubMed]
- Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer 2009;124:2406-15. [PubMed]
- Gong J, Hutter C, Baron JA, et al. A pooled analysis of smoking and colorectal cancer: timing of exposure and interactions with environmental factors. Cancer Epidemiol Biomarkers Prev 2012;21:1974-85. [PubMed]
- Rennert G, Rennert HS, Pinchev M, et al. Use of hormone replacement therapy and the risk of colorectal cancer. J Clin Oncol 2009;27:4542-7. [PubMed]
- Hoffmeister M, Raum E, Winter J, et al. Hormone replacement therapy, body mass, and the risk of colorectal cancer among postmenopausal women from Germany. Br J Cancer 2007;97:1486-92. [PubMed]
- Franceschi S, La Vecchia C. Colorectal cancer and hormone replacement therapy: an unexpected finding. Eur J Cancer Prev 1998;7:427-38. [PubMed]
- Kabat GC, Miller AB, Rohan TE. Oral contraceptive use, hormone replacement therapy, reproductive history and risk of colorectal cancer in women. Int J Cancer 2008;122:643-6. [PubMed]
- Purdue MP, Mink PJ, Hartge P, et al. Hormone replacement therapy, reproductive history, and colorectal adenomas: data from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial (United States). Cancer Causes Control 2005;16:965-73. [PubMed]
- Nanda K, Bastian LA, Hasselblad V, et al. Hormone replacement therapy and the risk of colorectal cancer: a meta-analysis. Obstet Gynecol 1999;93:880-8. [PubMed]
- Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med 2004;350:991-1004. [PubMed]
- Ritenbaugh C, Stanford JL, Wu L, et al. Conjugated equine estrogens and colorectal cancer incidence and survival: the Women’s Health Initiative randomized clinical trial. Cancer Epidemiol Biomarkers Prev 2008;17:2609-18. [PubMed]
- Chan AT, Giovannucci EL, Meyerhardt JA, et al. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA 2005;294:914-23. [PubMed]
- Chan AT, Giovannucci EL, Schernhammer ES, et al. A prospective study of aspirin use and the risk for colorectal adenoma. Ann Intern Med 2004;140:157-66. [PubMed]
- García-Rodríguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology 2001;12:88-93. [PubMed]
- Greenberg ER, Baron JA, Freeman DH Jr, et al. Reduced risk of large-bowel adenomas among aspirin users. The Polyp Prevention Study Group. J Natl Cancer Inst 1993;85:912-6. [PubMed]
- Logan RF, Little J, Hawtin PG, et al. Effect of aspirin and non-steroidal anti-inflammatory drugs on colorectal adenomas: case-control study of subjects participating in the Nottingham faecal occult blood screening programme. BMJ 1993;307:285-9. [PubMed]
- Tangrea JA, Albert PS, Lanza E, et al. Non-steroidal anti-inflammatory drug use is associated with reduction in recurrence of advanced and non-advanced colorectal adenomas (United States). Cancer Causes Control 2003;14:403-11. [PubMed]
- Gann PH, Manson JE, Glynn RJ, et al. Low-dose aspirin and incidence of colorectal tumors in a randomized trial. J Natl Cancer Inst 1993;85:1220-4. [PubMed]
- Flossmann E, Rothwell PM; British Doctors Aspirin Trial and the UK-TIA Aspirin Trial. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 2007;369:1603-13. [PubMed]
- Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 2003;348:891-9. [PubMed]
- Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med 2003;348:883-90. [PubMed]
- Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med 2006;355:873-84. [PubMed]
- Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med 2006;355:885-95. [PubMed]
- Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol 2012;13:518-27. [PubMed]
- Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA 2005;294:47-55. [PubMed]
- Stürmer T, Glynn RJ, Lee IM, et al. Aspirin use and colorectal cancer: post-trial follow-up data from the Physicians’ Health Study. Ann Intern Med 1998;128:713-20. [PubMed]
- Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376:1741-50. [PubMed]
- Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol 2009;10:501-7. [PubMed]
- Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011;378:2081-7. [PubMed]
- Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol 2005;23:2840-55. [PubMed]
- McQuaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am J Med 2006;119:624-38. [PubMed]
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71-86. [PubMed]
- U.S. Preventive Services Task Force. Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2007;146:361-4. [PubMed]
- Rothwell PM, Fowkes FG, Belch JF, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 2011;377:31-41. [PubMed]
- Chan AT, Arber N, Burn J, et al. Aspirin in the chemoprevention of colorectal neoplasia: an overview. Cancer Prev Res (Phila) 2012;5:164-78. [PubMed]


