Comprehensive clinical genetics care for patients with inherited colorectal cancer associated with Lynch syndrome: Western and Asian perspectives
Introduction
Although colorectal cancer (CRC) is a multifactorial disease, up to 20–30% of CRC patients have a family history of CRC, and up to 6% of cases have germline mutations that refer to an identifiable hereditary CRC syndrome (1).
The most common hereditary syndrome predisposing to CRC is Lynch syndrome (LS), which was first reported by Dr. Aldred S. Warthin more than 100 years ago (2). LS is an autosomal-dominant inheritance pattern syndrome and accounts for approximately 2–4% of all CRC cases (1 in 35 CRCs). LS results from germline mutations in the DNA mismatch repair (MMR) genes (MLH1, MSH2, MSH6, PMS2, being the four most major ones) or the EPCAM gene (located upstream from the MSH2 gene) (3). Since the 1980s, LS was called “hereditary nonpolyposis colorectal cancer” (HNPCC), because Dr. Lynch used the term to differentiate this syndrome from familial adenomatous polyposis (FAP), another CRC-predisposing inherited syndrome (4). However, the term HNPCC was somewhat misleading and a misnomer, because colorectal polyps or adenomas as well as extracolonic cancers have been recognized in patients with LS; therefore, by consensus the term “Lynch syndrome (LS)” has been more commonly utilized recently.
Distinguishing clinicopathologic features of LS
Individuals with LS face significantly elevated lifetime risks for CRC and extracolonic cancers when compared to general population risk, and they exhibit distinguishing clinic-pathologic features that are unique from patients with sporadic CRC or of other hereditary syndromes.
Lifetime risk of CRC
LS is associated with a lifetime risk of 30–70% for CRC, depending on gender and mutation of the MMR gene (5). The most significant risks are associated with MLH1 and MSH2. CRCs can arise as rapidly as within 3 years after a clearing colonoscopy (6). Indeed, in known LS families, the risk for CRC is 4.1% in 5 years and 8.1% in 10 years for yet-unaffected carriers (median age of 49 years), as compared to 0.39% at 5 years and 2.4% at 10 years for non-carriers (7).
Young age of onset
The mean age of diagnosis for the first CRC in patients with LS ranges between 44–61 years, significantly earlier than the mean age of diagnosis of 69 years for sporadic CRC in the Western world. This young-onset presentation may be related to a more rapid adenoma-carcinoma sequence in LS of about 35 months as compared to approximately 7–10 years in sporadic CRC cases (8). Among patients with CRC diagnosed before age 35, LS can account for up to 35% of cases (9). This proportion is significantly higher than the 4–6% (1) among all CRCs diagnosed at all ages.
Proximal colon predominance
Approximately 60–80% of CRC cases with LS arise in the right side of the colon, proximal to the splenic flexure, compared with 30% of sporadic CRC; only 15–20% of the cases present as rectal cancer (8). Therefore, colonoscopy, including viewing of the cecum, is the modality of choice for endoscopic screening and surveillance of CRC in LS patients.
Distinctive histopathology
LS-associated CRC more frequently exhibit microscopic features including mucinous/signet cell histology, poor differentiation, and evidence of tumor-infiltrating lymphocytes. These are hallmarks of microsatellite instability (MSI) status, and a hypermutated phenotype (10). This phenotype is associated with a high degree of immunogenicity.
Better prognosis
Patients with CRC in the setting of LS experience a better prognosis than those with sporadic CRCs. A cohort analysis of stage III CRC found that 5-year overall survival was significantly more favorable in LS patients than in patients with sporadic disease, 70% vs. 56%, respectively (11). Another study reported 5-year cumulative survival rates of 60–94.2% in LS patients, compared with 52–75.3% in sporadic CRCs (12). Limited evidence suggests that MSI-high CRCs may have a different recurrence pattern that MSS CRCs, with more frequent local or peritoneal recurrence (13).
Synchronous and metachronous adenomas and adenocarcinomas
LS is associated with early-onset oligopolyposis. In a recent retrospective study of 263 patients with LS, 41% of patients had at least one adenoma, including 11% with 2 to 5 adenomas and 4% with 10 or more cumulative adenomas (14). Similarly, patients with LS can present with synchronous pathologies, such as synchronous advanced adenomas, synchronous adenoma and invasive adenocarcinoma, or multiple adenocarcinomas. Metachronous CRC is defined as a separate primary CRC occurring more than 6 months after surgical resection of the index CRC (3). The risks for metachronous CRC after segmental colon resection of the index CRC have been reported as: 16% at 10 years, 41% at 20 years, and 62% at 30 years (15).
Extracolonic malignancies
Individuals with LS are also predisposed to a wide variety of extracolonic cancers. Women with LS have significantly increased (20% to 60%) lifetime risk for endometrial cancer (EC) (16). Other common cancer sites associated with LS include the urologic tract, stomach, ovary, hepatobiliary tract, small bowel, and pancreas. Association of LS with prostate, breast, and lung cancers has also been reported (6,8,17) (Table 1).
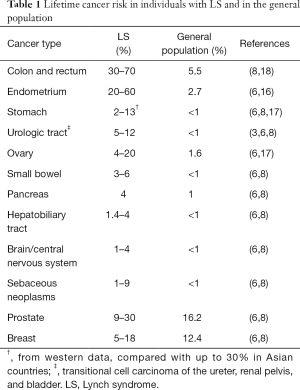
Full table
Mutation dependent
The risks of CRC and extracolonic cancer in carriers vary depending on the different germline mutation in the MMR gene. The cumulative cancer risks of LS were similar in MLH1 and MSH2 mutation carriers (59% and 57%), but significantly lower in mutation carriers of MSH6 gene (10–25%) (18) and PMS2 gene (15–20%) (19). Additionally, MLH1 mutation carriers tend to develop CRC at younger ages, compared with MSH2 carriers who seem to be at higher risk for rectal cancers (20) and extracolonic cancers, such as EC (48%) and urothelial cancer (80%) (18,21). MSH6 gene mutation carriers seem to have lower CRC risk, with disease tending to occur at later ages, compared with substantially higher risk (71%) in women with EC (22). It also reported a lower PMS2 mutation penetrance for CRC and EC compared with other gene mutation carriers (23).
Diagnosis of LS through tumor molecular testing and germline mutation testing
Making the diagnosis of LS is critical because a missed diagnosis means overlooking the dangers of high lifetime cancer risks in mutation carriers. Early detection and diagnosis can be aided by screening CRC tumor tissue for evidence of hypermutability or MSI, through somatic testing by immunohistochemistry (IHC) staining and/or MSI testing. In addition to screening, the diagnosis of LS can be confirmed by the identification of a germline mutation in one of the major MMR genes or the EPCAM gene (1).
Tumor molecular testing: IHC and MSI testing
One of the most significant discoveries in the last century concerning LS is the recognition that MSI is the molecular signature of a defective DNA MMR mechanism. Nearly all of LS-associated CRCs display a high level of MSI, also termed MSI-high (or MSI-H) (3).
MSI is thought to arise from defects in the DNA MMR system. If the wild-type allele in MMR genes is lost or inactivated, the gene product MMR protein can no longer repair DNA mismatch errors that inevitably arise during DNA replication, leading to accumulation of mutations in the DNA. One of the places in the tumor DNA where such errors become most manifest is at simple repetitive sequences (microsatellites). Therefore, identifying disruptions in repetitive sequences in the tumor DNA, but not in the adjacent normal tissue, is the gold-standard method for detecting MSI (24). MSI testing is a polymerase chain reaction (PCR) based test that tests for allelic shift in a standardized panel of markers, most typically BAT25, BAT26, BAT40, D5S346, D2S123, D17S250, and TGFBR2. By consensus, MSI-high is defined by 30% or more of the markers in the panel showing allelic shift, while MSI-stable (MSS) is defined by 0% of the markers showing allelic shift, and the remaining is defined as MSI-low (25).
IHC can be performed on tumor tissue to detect the presence or absence of MMR proteins. Deficient expression of MMR proteins indicates an underlying defect in the MMR genes. Typically, MMR proteins combine as heterodimer complexes, such as “MLH1-PMS2” or “MSH2-MSH6”, and work synergistically to target microsatellite repair. Therefore, typical IHC staining pattern can show loss of expression of MLH1 with PMS2, and loss of expression of MSH2 with MSH6 when there is underlying defect in MLH1 or MSH2 genes respectively (26).
MSI arising from epigenetic silencing of MLH1
MSI-high or hypermutator phenotype is observed in about 12–15% of all CRCs (10). The majority of MSI-high CRCs arise not from germline mutations in MMR genes, but from other causes. The most well-known of these is epigenetic silencing of the MLH1 gene caused by hypermethylation of the MLH1 promoter (8). MLH1 promoter methylation turns off the production of MLH1 mRNA and protein product, leading to loss of MLH1 expression on IHC testing (27). Additionally, this hypermethylation is often associated with a BRAF c.1799T > A (p.V600E) mutation (28). Therefore, when IHC shows absence of MLH1/PMS2 protein expression, the analysis of MLH1 promoter hypermethylation and/or BRAF V600E mutation should be conducted. Rarely, such hypermethylation can be inherited. In a series of 331 LS-suspected patients, the frequencies of germline MLH1 promoter hypermethylation was 0.6% (29).
Germline mutations in major MMR genes and EPCAM
LS is caused by germline mutations in at least one of four DNA MMR genes, MLH1, MSH2, MSH6, and PMS2. The MMR proteins work to maintain genomic fidelity by identifying and correcting replication errors (single nucleotide mismatch or insertion and deletion loops) that have escaped the normal editing function of DNA polymerase (30).
According to the study of Dr. Lynch in 2003, two main genes, MLH1 and MSH2, account for almost 90% of all identified mutations, with only 10–12% of the mutations in the MSH6 gene and 1–2% in the PMS2 gene (3). However, recent publications have indicated that the frequencies of MSH6 and PMS2 gene mutations have likely been underestimated in the past, because affected carriers display less striking phenotypes. The International Society for Gastrointestinal and Hereditary Tumours (InSiGHT) database and other population-based studies showed that LS-associated mutations are likely comprised of: 35–42% in MLH1, 33–44% in MSH2, 8–18% in MSH6, and 2–7.5% in PMS2 (31).
EPCAM gene is upstream from MSH2. The deletion of the 3’ terminal codon of EPCAM, previously called TACSTD1, leads to hypermethylation and silencing of MSH2, leading to a phenotype similar to that of LS (32). Thus, mutation testing in the EPCAM gene should be considered in cases of absent expression of MSH2 and/or MSH6 by IHC.
Guidelines for diagnosing LS among CRC patients: Western and Asian perspectives
The early identification of LS is critical for effective cancer prevention in probands and their at-risk family members. Historically, the diagnosis of LS relied on pedigree features such as clustering of cancers at early ages. However, diagnostic approaches to LS have significantly evolved over the past 100 years. In 1991, the International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer established the Amsterdam I criteria (Table 2) for HNPCC (33). By 1999, these criteria were replaced by the Amsterdam II criteria (Table 2), to include some extracolonic tumors (except gastric and ovarian cancer) as qualifying criteria for LS (34). Amsterdam I/II criteria rely on an accurate and detailed family history, but neither set is optimal due to limited sensitivity and specificity. Unfortunately, 40% of families with known MMR mutations do not fulfill the Amsterdam criteria. On the contrary, 50% who do meet the criteria have no detectable MMR gene deficiency (“Syndrome X”) (21). In 2004, a third set of clinical criteria, the revised Bethesda guidelines (35) (Table 2), were developed to encourage evaluation of MSI-high status in CRC tumors by MSI and/or IHC, to identify individuals who should undergo further genetic testing for LS. This provides a screening approach with higher sensitivity (approximately 70%) (4). There are several limitations to replying on clinical criteria alone. A comprehensive family history is often not available for every cancer patient, and family size has decreased over time, making pedigrees less informative. Thus, current guidelines for LS diagnosis utilize not only family history but also molecular testing, germline mutational analysis, and clinical prediction models.

Full table
We summarized most currently published evidence-based guidelines for LS diagnosis (Table 3), and discuss their differences from the Asian and Western perspectives (6,8,36-39).
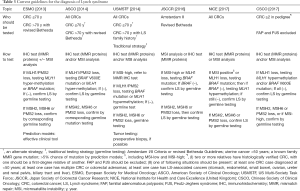
Full table
Who should be tested?
The guidelines differ depending on whether the individual is clinically affected by CRC or whether a germline mutation is known in the family (40). The remaining discussion pertains to testing of individuals presenting with a CRC without a known family mutation. Current guidelines around the globe remain divided in terms of selective testing or universal testing of CRC patients for LS. More recent guidelines from Western countries advocate a universal testing approach, while still acknowledging alternative strategies that are more selective. On the other hand, the older European guideline and the available guidelines from Asia have taken the selective testing approach (Table 3).
Selecting who to test based on clinical criteria outlined in Table 2 has been criticized for low sensitivity and efficiency. Meanwhile, several clinical prediction models that take into account clinical, family history and tumor MSI status information have been developed, including MMRpredict (41), MMRpro (42), and PREMM1, 2, 6 (43). These computational models aim to estimate the probability of finding a pathogenic mutation in a MMR gene. The overall sensitivity and specificity of these models have been reported to be up to 90% (4). Unfortunately, practical utilization of these models has remained limited by lack of awareness among physicians and lack of compliance by suspected patients. During the Jerusalem workshop organized by Dr. Shike in 2009, selective testing of CRC patients who are younger than 70 years of age was proposed as more cost-effective than universal testing, while missing only 4.9% of LS cases (44). Hence, selective universal screening, testing all CRCs diagnosed at age ≤70 years and selective screening in patients with CRC diagnosed at age >70 years who meet revised Bethesda guidelines, has been adopted by some guidelines (6,36).
Universal testing provides a screening strategy among newly diagnosed CRC patients with 100% sensitivity. A pooled analysis demonstrated that relative to universal testing, the relative sensitivity for selective testing based on the Bethesda guidelines was 87.8%, on Jerusalem recommendations was 85.4%, and the selective universal screening (95.1%), with similar specificities (45). However, a more recent study found that 18% of LS cases had CRC diagnosed above age 70 years (46). As more guidelines support universal testing in all newly diagnosed CRC cases regardless of age (8,37), attention should be paid to the impact on healthcare costs, patient anxiety, and the feasibility of widespread adoption.
How to test?
Although almost all recent guidelines for LS utilize tumor-based IHC and MSI testing as well as germline testing, subtle differences exist (Table 3). For example, both the 2013 European Society for Medical Oncology (ESMO) and the 2014 the American Society of Clinical Oncology (ASCO) guidelines recommended that CRC tumors be tested by IHC for MMR proteins and/or MSI (6). ASCO has emphasized that IHC testing has advantages in identifying loss of specific MMR protein expression, which may help target confirmatory germline testing toward the MMR gene most likely to be mutated. Secondly, the US Multi-Society Task Force (USMSTF) stated in 2014 that if a tumor demonstrates loss of MLH1, either BRAF mutation testing “or” analysis of MLH1 promoter hypermethylation should be performed (8). However, 30% to 50% of CRCs can present as BRAF wild-type and still have MLH1 promoter hypermethylation (47). Therefore, the National Institute for Health and Care Excellence (NICE) in the United Kingdom emphasizes that, if the result for MLH1 is abnormal, sequential BRAF V600E “and” MLH1 promoter methylation testing should be performed (37).
Emerging use of multi-gene panel testing
With advances in genomic medicine and next-generation sequencing (NGS), multiple genes can be sequenced using panel testing. When compared to traditional phenotype-directed germline testing, multi-gene panel testing can identify individuals who have hereditary cancer syndrome with atypical and even no, phenotypes or clinical presentations (48). It can test multiple genes simultaneously and is more efficient when there is a wide differential diagnosis for the potential hereditary syndromes. There is wide variation in the exact nature and composition of the available panels. Some include not only highly penetrant genes that predispose mutation carriers to CRC (e.g., APC, MUTYH, MMR genes, and SMAD4) but also low penetrance genes or genes related to malignancies other than CRC (e.g., PTEN, CDH1, STK11, BRCA1, and BRCA2) (49). Therefore, the main challenge of clinical use of these panels is the increased complexity of result interpretation and assessment of the clinical significance of uncertain or unexpected findings. At present, the United States National Comprehensive Cancer Network (NCCN) guidelines do not recommend routine use of multi-gene testing as a universal testing strategy. And the need for careful case selection under the direction of a clinician with expertise in genetics is emphasized (48).
“Lynch-like syndrome” (LLS) or “mutation-negative” LS
Germline testing for LS can lead to three categories of results: pathogenic mutation, variant of uncertain significance (VUS), and informative negative finding (50). The practice of universal tumor MSI testing followed by confirmatory germline mutation testing has led to identification of a cohort of patients who present with MSI-high CRC, no evidence of MLH1 promoter hypermethylation or BRAF mutation, but have no identified pathogenic mutation on germline testing (51).
Unclassified variants were reported to account for 20–50% of all those tested for MMR mutations (51). The use of multiplex gene panel testing is associated with even higher proportions of variants of uncertain significance and uninformative negative results would be observed. Currently, these variants are thought to potentially represent missense mutations (52), which would cause unknown or highly variable clinical effects. There is a significant amount of active research in variant reclassification. These efforts utilize a combination of in silico prediction models, in vitro functional assays, and aggregate clinical data. However, translation of variant reclassification approaches to clinical practice is challenging.
Among patients with uninformative negative germline testing results, a proportion harbor what has been termed “Lynch-like” LS or “mutation-negative” LS (51,53-55). These patients behave similarly to LS patients, but they show lower risks for malignancy: the standardized incidence ratio (SIR) for CRC in LLS vs. LS is 2.12 vs. 6.04, and for extra-colonic cancers in LLS vs. LS is 1.69 vs. 2.81. The molecular mechanism for LLS has not been fully elucidated. A subset of these patients has been found to harbor two somatic events that affect MMR gene expression. Examples may include a somatic mutation coupled with loss of heterozygosity (LOH), or two somatic biallelic mutations of DNA MMR genes. In a series 1,234 newly diagnosed CRC patients from Japan who underwent universal tumor MSI testing, 11 (0.9%) were referred for confirmatory germline testing. Pathogenic mutation was found in 9 patients, while 2 (0.2%) remaining patients harbored biallelic somatic deletion of MMR genes and uninformative germline test result. The authors suggest that the prevalence of pathogenic mutations for LS among CRC patients in Japan appeared similar to that reported by studies in Western population, but the prevalence of LLS was extremely low (56).
The clinical management of these patients discussed above is controversial. It appears prudent to continue to perform surveillance for cancer development, while clinical data continue to accumulate. However, it is likely that following the same surveillance guidelines as those for LS represents overtreatment.
Clinical genetics care for CRC patients with LS
Patients who present with CRC on the background of LS require tailored and personalized clinical genetic care. Clinical genetics care should focus on both the treatment of the CRC as well as the prevention of future CRC and extracolonic cancers; additionally, needs of both the proband as well as his/her at-risk relatives should be addressed. Preventive strategies include prophylactic surgery, active comprehensive surveillance, as well as chemoprevention.
Multidisciplinary oncology treatment of CRC in patients with LS
Surgical intervention
Surgical treatment of CRC in patients with LS should be planned to provide optimal oncologic treatment of curative-intent for the index CRC, while also taking into account the high risks of metachronous high risk adenoma or CRC as well as for other extracolonic cancers in the patient (57).
Oncologic resection of the index CRC should include segmental resection with adequate locoregional lymphadenectomy. For LS patients presenting with an index colon cancers, the risk of developing a metachronous high-risk adenoma or invasive cancer is significant as previously discussed. Therefore, subtotal or total abdominal colectomy has been advocated over segmental colectomy to offer the advantage of decreased risks of metachronous lesions (58). Studies that have compared LS patients who underwent segmental colectomy vs. total colectomy have reported that 25–26% developed a second CRC at a median follow-up of 5.7 years, compared with 6–8% after total colectomy (59). High-risk adenomas were found in 22%. However, from a survival view, there is no convincing evidence that more extensive surgery improves overall survival in LS patients when compared to segmental resection and colonoscopic surveillance, acknowledging that the latter strategy might endure more short-interval colonoscopies and even repetitive surgery. In a recent study (60), subtotal colectomy decreased the risk of subsequent CRC [hazard ratio (HR) 0.20; 95% CI: 0.08–0.52] compared with segmental resection, but there was no difference in overall survival nor in disease-specific survival, between the standard and extended surgeries (47.2% vs. 41.4%, P=0.83; and 82.7% vs. 87.2%, P=0.76) within 25 years. Another study also demonstrated similar results (57). Finally, it is important to consider that the functional outcomes of segmental colectomy differ significantly from those of total abdominal colectomy (61). After total abdominal colectomy, the frequency of stool can be as high as 4 to 6 times daily; over 50% of the patients reported dietary restriction; and over 30% reported restriction of social activity and recreation when compared to preoperative levels (61).
For LS patients presenting with an index rectal cancer, the choice of operation is even more controversial. In the largest series of rectal cancers in patients with LS, the extent of surgical resection was influenced by synchronous colonic disease (i.e., adenomas) at presentation, tumor height, clinical stage, and pelvic radiation (20). Similar to the case in colon cancer, limited resection focusing on proctectomy did not compromise overall survival when compared to total proctocolectomy.
Therefore, the optimal surgical decision would be an informed and balanced choice for the individual LS patient, considering the risks and benefits for limited or extended procedures. Extensive resections minimize the risks for metachronous lesions but typically carry functional sequela without significant survival advantages (62,63). Higher compliance and more frequent lifetime endoscopic surveillance of any remaining colorectum are required for patients offered limited resection.
Systemic therapy including chemotherapy and immunotherapy
Common chemotherapy regimens for CRC include fluorouracil (5-FU), oxaliplatin-based (FOLFOX), and irinotecan-based (FOLFIRI). However, the efficacy of single agent 5-FU in MSI-high CRCs associated with LS is controversial. In in-vitro studies, MMR-deficient colon cancer cells had an approximately 18-fold increase in resistance to 5-FU, compared with MMR-proficient cells (64). Another study revealed a strong correlation between MMR deficiency and 5-FU resistance in a panel of 77 CRC cell lines (65). In addition, single agent 5-FU is ineffective in MMR-deficient CRC patients with stage II disease in the adjuvant setting (66). Finally, single agent 5-FU appeared to be an appropriate chemo-sensitizing agent for rectal cancer in the setting of neoadjuvant chemoradiation (20).
For patients with metastatic CRC, FOLFOX-based regimens have generally been thought to be effective, although a small study suggested that MMR-deficient CRCs may show a lower treatment response rate compared with MMR-proficient CRCs (11.7% vs. 28.6%) (67). Most recently, MSI-high phenotype has been found to be highly immunogenic with excellent response to immunotherapy agents, such as checkpoint inhibitors (68). MSI-high CRCs express high levels of checkpoint proteins, including programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1) (69). Inhibition of the interaction of PD-1 and/or PD-L1 checkpoint proteins allows the innate anticancer system and immune T cells to remain active and attack malignant cells (70). A phase II clinical trial treated patients with progressive metastatic solid tumors with pembrolizumab, an anti-PD-1 immune checkpoint inhibitor. Patients with MMR-deficient CRC showed significantly higher rates of objective response (40% vs. 0%) and progression-free survival (78% vs. 11%), when compared with patients with MMR-proficient CRC (71). In the more recent phase II CheckMate-142 trial, anti-PD-1 monoclonal antibody, nivolumab, demonstrated excellent response and disease control in histologically confirmed recurrent or metastatic CRC with MMR deficiency (72). After a median follow-up of 12 months, objective response rate was 31.1% and the overall survival rate was 73.8%, with a median progression-free survival of 9.6 months. Compared with chemotherapy, immunotherapy agents have shown significantly less toxicity rates. Additional clinical trials involving immunotherapy are ongoing.
Radiotherapy
Radiation activates DNA-damage responses that may lead to apoptosis of cancer cells (73). While some in vitro studies reported that MMR deficiency was associated with sensitivity to ionizing radiation in CRC cells (74), others suggest that they showed more resistance to low-dose irradiation (75). The clinical studies, however, have mainly supported the effectiveness of radiation in MSI-high CRCs. In a series of rectal cancers treated with neoadjuvant chemotherapy (5-FU and CPT-11) and radiotherapy (45 to 54 Gy), MMR-deficient rectal cancers showed a 60% complete response (76). In our series of 62 MMR-deficient rectal cancers, 30 (75% of 40 patients with clinical stage II or III disease) received neoadjuvant 5-FU based chemoradiation. The complete pathologic response rate was 27.6%, and 55.5% were downstaged. After a median follow-up of 6.8 years, the 5-year disease-specific survival rates were 100% for stage I and II, 85.1% for stage III, and 60.0% for stage IV disease (20). Radiotherapy has also been routinely incorporated in the treatment of LS-associated extracolonic cancers, such as endometrial and brain cancers.
Life-long multi-organ surveillance in LS patients
Screening for LS-associated CRC by colonoscopy is universally recommended (8) (Table 4). Screening should be performed every 1–2 years starting around age 20–25 years, or 2–5 years younger than the CRC diagnosis of the youngest CRC family member if he or she was diagnosed earlier than 25 years old. Colonoscopy screening has been shown to increase life expectancy, reduce CRC incidence by 62% (77), and identify CRC at earlier stages. The risks of CRC differ by different MMR gene mutations: MSH6 and PMS2 mutation carriers have a lower risk of CRC and older age at diagnosis than do patients with MLH1 and MSH2 mutations (22). Therefore, some have suggested that screening for these individuals could be delayed until age 30 in MSH6 and 35 in PMS2 carriers, unless an early-onset cancer exists in the family history (8). However, when implementing surveillance management in LS cases, many challenges still exist, including patient compliance, endoscopist availability, and access to care (78).

Full table
Surveillance of the remaining colon and rectum in LS who has had a limited resection for an index CRC is critical for triggering timely management of any metachronous pathology (79).
Screening guidelines for extracolonic cancers have remained controversial, mainly due to the lack of evidence for survival benefit and the lack of consensus. For example, the optimal surveillance strategy for EC has not been determined. The ESMO guidelines recommend gynecologic examination, pelvic ultrasound, cancer antigen 125 (CA-125), and endometrial aspirate annually beginning at age 30 to 35 years (36). But this is not endorsed by other societies who consider the evidence for routine surveillance for endometrial and ovarian cancers limited (6) (Table 4).
Chemoprevention
Aspirin
The largest clinical trial of chemoprevention for LS-associated CRC is the CAPP2 trial (80), launched by the Colorectal Adenoma/Carcinoma Prevention Program (CAPP) in 1999. Mutation carriers were randomized in a 2×2 design to aspirin (600 mg daily vs. placebo) and resistant starch (starch vs. placebo) After long-term follow-up of 55.7 months, LS patients treated with aspirin for ≥2 years showed a HR of 0.41 for CRC and of 0.45 for any LS-related cancer when compared to placebo (81). However, it is notable that the optimal aspirin dose, duration of use, and associated side effects require further research. Therefore, the CAPP3 trial is currently ongoing. It aims to randomize 1,000 LS mutation carriers to 3 doses of aspirin (100, 300, and 600 mg daily), and will examine the CRC incidence and adverse event rate during the 5- to 10-year follow-up period (68). In addition, another clinical trial (AAS-Lynch) will evaluate the effect of low-dose aspirin (100 mg daily vs. 300 mg daily) on colorectal adenomas formation in patients with LS.
Naproxen
Naproxen is a non-steroidal anti-inflammatory drug (NSAID) with minimal cardiac side effects. A randomized phase Ib/II clinical trial (NCT02052908) is investigating the effects of naproxen in preventing CRC in MMR-deficient (LS) patients. Patients receive high-dose naproxen, low-dose naproxen, or placebo for 6 months, respectively (69). This study will compare prostaglandin E2 (PGE2) concentration levels in normal colorectal mucosa at different doses.
Unique aspects of LS diagnosis and management in Asia
Although Asia is the most populous continent, knowledge regarding LS-associated CRC in Asian countries has been limited. Based on available reports, the clinical manifestations similarly highlight features such as early onset of CRC, more aggressive histopathology, lifetime cancer risk, and multiple extracolonic cancers. However, a few unique aspects warrant highlighting.
Lower prevalence or incidence of LS?
The prevalence of LS in Asian has been thought to be lower than that reported in Western populations, where the prevalence rate is estimated at 3–5%, depending on the ascertainment method. In a retrospective study of Chinese CRC patients, LS, as defined by meeting the Amsterdam criteria I or II, was found in 1.24% and 2.15% of the patients, respectively (70). Another Japanese study of 452 patients with CRCs, LS, as defined by pathogenic germline mutation in MLH1 or MSH2 was found in only 1.7% (71). In a recent study of Japanese CRC patients under the age of 60 years, using MSI testing and IHC as a primary screening method, only 2.2% of the CRCs were found to be suggestive of LS (72). Furthermore, several studies demonstrated that the incidence rates of synchronous and metachronous cancer in Chinese LS patients ranges between 10.0–20.4% (82), compared with substantially higher rates (16–62%) in Western studies (15).
It should be noted that using the clinical criteria (Amsterdam I/II) to ascertain LS in China is likely inaccurate due to under-estimation because of the universally small family sizes in China arising from the national “one-child policy.” Therefore, a modification of the original criteria has been proposed to “at least two pathologically verified CRCs in a family; at least two are first-degree relatives including parents or siblings” (83). In addition, the algorithm of germline testing has mainly only included MLH1 and MSH2 in Asian studies (84), and the lack of testing of MSH6, PMS2, and EPCAM likely account for some degree of underestimation (85,86).
High risks of gastric cancer?
Another significant difference in Asia is the high prevalence of gastric cancer among extracolonic malignancy associated with LS. In a study of 30 LS families diagnosed by the Amsterdam criteria, 37% extracolonic malignancies were gastric cancer, while only 4 (13.3%) cases were EC (87). Another study including both Chinese and Korean LS families also found that gastric cancer was the second most common cancer in these families (88). Moreover, among 98 cancer deaths in female first-degree relatives from Japanese LS families, 53%, 19%, and 13% of the deaths were due to colorectal, gastric, and uterine cancers, respectively (89). Similar results were reported in other studies (90). Compared with only a 2–13% lifetime risk in Western countries (8), the risk of gastric cancer in Asian studies is theoretically estimated at approximately 30% in LS probands for MMR mutation carriers (91,92). Consequently, surveillance focusing on the upper gastrointestinal tract has been considered for Asian patients.
Preferential sidedness?
While European and American studies have highlighted a predominance for proximal location for LS-associated CRCs, this has been controversial in studies from Asian populations.
One study reported that among tumors in patients who meet the Amsterdam criteria, 69% were left sided, with most being sigmoid cancers (93). In another study from China, 31 families demonstrated a remarkably high proportion of left-sided CRC (60.6%) and a lower synchronous cancer incidence (8.5%), compared with Western data (90). In a recent Chinese study of 116 patients with LS, more left-sided and rectal tumors were found (64.7% vs. 35.3% right-sided) (94). Other studies have reported that LS-associated CRCs were proximal to the splenic flexure in only 40.9% (95) or only 39% (96).
However, the reported proportions of CRCs arising from right vs. left side may reflect recruitment bias. At least one study of 34 LS families in China found 77 (66.4%) of the cases developed in the proximal colon, and 39 (33.6%) cases were distal colon cancer, a pattern not dissimilar to that reported in Western countries (97). It should also be noted that greater than 50% of all CRCs diagnosed in China or other Asian countries in general are rectal cancer (83), and may represent a baseline difference in population. Hence, whether the prominent left-sided feature found in Asia indicates a potential recruitment bias or an accurate phenomenon requires more evidence.
Clinical genetics care guidelines of LS in Asia
Given the above distinct features of LS, it is worthwhile to compare the clinical care guidelines from Asian vs. Western countries (Table 4). Several differences exist. First, while the debate between segmental vs. extended resection exist for management of index CRC associated with LS, the Asian guidelines do not strongly advocate for extended resection. Neither extensive colectomy for index CRC nor prophylactic surgery for unaffected carriers is advocated, and are not mentioned in the current Chinese Society of Clinical Oncology (CSCO) guidelines (39). Indeed, in a Japanese study, extensive surgery (proctocolectomy/colectomy) was preferred by only 9% of the care providers, and prophylactic gynecologic surgery was preferred by only 18% for postmenopausal women (98). Secondly, Asian guidelines have strongly recommend screening with upper gastrointestinal endoscopy, for example, every 1–2 years in Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines (38), despite firm evidence of survival benefit. This is in contrast with the Western guidelines that would consider esophagogastroduodenoscopy every 2–3 years based on significant patient and/or family risk factors, while acknowledging that the evidence base for the consideration is low (8). Finally, strategies for chemoprevention have largely been absent in the Asian guidelines. This likely reflects the lack of chemoprevention trials in Asian populations, where only one Chinese study (99) reported the use of celecoxib, another NSAID, in patients with LS and FAP. After 9-month treatment with celecoxib at 400 mg/d, the polyps vanished in most patients with LS, but side effects were observed, including arrhythmia, angina pectoris, and nervous headache.
Conclusions
LS, caused by inheritable deficiency in the DNA MMR system, predisposes patients to CRC and extracolonic cancers. Improved understanding of the molecular basis of LS has enabled universal tumor-based screening, genetic counseling, and advanced germline testing. Finally, a system for comprehensive clinical genetics care should be established to provide optimal treatment of the index CRC, as well as implementation of preventive strategies include prophylactic surgery, active comprehensive surveillance, as well as chemoprevention. Increased international exchange and collaboration is needed to help reduce the global burden of inherited colorectal and extracolonic malignancies associated with LS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Herzig DO, Buie WD, Weiser MR, et al. Clinical Practice Guidelines for the Surgical Treatment of Patients With Lynch Syndrome. Dis Colon Rectum 2017;60:137-43. [Crossref] [PubMed]
- Boland CR, Lynch HT. The history of Lynch syndrome. Fam Cancer 2013;12:145-57. [Crossref] [PubMed]
- Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003;348:919-32. [Crossref] [PubMed]
- Lynch HT, Snyder CL, Shaw TG, et al. Milestones of Lynch syndrome: 1895-2015. Nat Rev Cancer 2015;15:181-94. [Crossref] [PubMed]
- Weissman SM, Bellcross C, Bittner CC, et al. Genetic counseling considerations in the evaluation of families for Lynch syndrome--a review. J Genet Couns 2011;20:5-19. [Crossref] [PubMed]
- Stoffel EM, Mangu PB, Gruber SB, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol 2015;33:209-17. [Crossref] [PubMed]
- Win AK, Young JP, Lindor NM, et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J Clin Oncol 2012;30:958-64. [Crossref] [PubMed]
- Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. Am J Gastroenterol 2014;109:1159-79. [Crossref] [PubMed]
- Mork ME, You YN, Ying J, et al. High Prevalence of Hereditary Cancer Syndromes in Adolescents and Young Adults With Colorectal Cancer. J Clin Oncol 2015;33:3544-9. [Crossref] [PubMed]
- Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015;21:1350-6. [Crossref] [PubMed]
- Brixen LM, Bernstein IT, Bulow S, et al. Survival of patients with Stage III colon cancer is improved in hereditary non-polyposis colorectal cancer compared with sporadic cases. A Danish registry based study. Colorectal Dis 2013;15:816-23. [Crossref] [PubMed]
- Stigliano V, Assisi D, Cosimelli M, et al. Survival of hereditary non-polyposis colorectal cancer patients compared with sporadic colorectal cancer patients. J Exp Clin Cancer Res 2008;27:39. [Crossref] [PubMed]
- Kim CG, Ahn JB, Jung M, et al. Effects of microsatellite instability on recurrence patterns and outcomes in colorectal cancers. Br J Cancer 2016;115:25-33. [Crossref] [PubMed]
- Kalady MF, Kravochuck SE, Heald B, et al. Defining the adenoma burden in lynch syndrome. Dis Colon Rectum 2015;58:388-92. [Crossref] [PubMed]
- Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 2011;60:950-7. [Crossref] [PubMed]
- Lynch HT, Shaw TG. Practical genetics of colorectal cancer. Chin Clin Oncol 2013;2:12. [PubMed]
- Douglas JA, Gruber SB, Meister KA, et al. History and molecular genetics of Lynch syndrome in family G: a century later. JAMA 2005;294:2195-202. [Crossref] [PubMed]
- Bonadona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011;305:2304-10. [Crossref] [PubMed]
- Goodenberger ML, Thomas BC, Riegert-Johnson D, et al. PMS2 monoallelic mutation carriers: the known unknown. Genet Med 2016;18:13-9. [Crossref] [PubMed]
- de Rosa N, Rodriguez-Bigas MA, Chang GJ, et al. DNA Mismatch Repair Deficiency in Rectal Cancer: Benchmarking Its Impact on Prognosis, Neoadjuvant Response Prediction, and Clinical Cancer Genetics. J Clin Oncol 2016;34:3039-46. [Crossref] [PubMed]
- Kastrinos F, Stoffel EM. History, genetics, and strategies for cancer prevention in Lynch syndrome. Clin Gastroenterol Hepatol 2014;12:715-27; quiz e41-3.
- Baglietto L, Lindor NM, Dowty JG, et al. Risks of Lynch syndrome cancers for MSH6 mutation carriers. J Natl Cancer Inst 2010;102:193-201. [Crossref] [PubMed]
- Blount J, Prakash A. The Changing Landscape of Lynch Syndrome due to PMS2 Mutations. Clin Genet 2017. [Epub ahead of print].
- Aaltonen LA, Peltomaki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993;260:812-6. [Crossref] [PubMed]
- Pawlik TM, Raut CP, Rodriguez-Bigas MA. Colorectal carcinogenesis: MSI-H versus MSI-L. Dis Markers 2004;20:199-206. [Crossref] [PubMed]
- Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology 2008;135:1079-99. [Crossref] [PubMed]
- Cunningham JM, Kim CY, Christensen ER, et al. The frequency of hereditary defective mismatch repair in a prospective series of unselected colorectal carcinomas. Am J Hum Genet 2001;69:780-90. [Crossref] [PubMed]
- Deng G, Bell I, Crawley S, et al. BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin Cancer Res 2004;10:191-5. [Crossref] [PubMed]
- Niessen RC, Hofstra RM, Westers H, et al. Germline hypermethylation of MLH1 and EPCAM deletions are a frequent cause of Lynch syndrome. Genes Chromosomes Cancer 2009;48:737-44. [Crossref] [PubMed]
- Buermeyer AB, Deschenes SM, Baker SM, et al. Mammalian DNA mismatch repair. Annu Rev Genet 1999;33:533-64. [Crossref] [PubMed]
- Plazzer JP, Sijmons RH, Woods MO, et al. The InSiGHT database: utilizing 100 years of insights into Lynch syndrome. Fam Cancer 2013;12:175-80. [Crossref] [PubMed]
- Ligtenberg MJ, Kuiper RP, Chan TL, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3' exons of TACSTD1. Nat Genet 2009;41:112-7. [Crossref] [PubMed]
- Vasen HF, Mecklin JP, Khan PM, et al. Amsterdam I criteria:The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum 1991;34:424-5. [Crossref] [PubMed]
- Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999;116:1453-6. [Crossref] [PubMed]
- Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261-8. [Crossref] [PubMed]
- Balmaña J, Balaguer F, Cervantes A, et al. Familial risk-colorectal cancer: ESMO Clinical Practice Guidelines. Ann Oncol 2013;24 Suppl 6:vi73-80. [Crossref] [PubMed]
- NICE. Molecular testing strategies for Lynch syndrome in people with colorectal cancer, Diagnostics guidance [DG27]. 2017.
- JSCCR. JSCCR (Japanese Society for Cancer of the Colon and Rectum) Guidelines 2016 for the Clinical Practice of Hereditary Colorectal Cancer. 2016.
- CSCO. Chinese Society of Clinical Oncology (CSCO) Guidelines for Hereditary Colorectal Cancer. 2017.
- Tiwari AK, Roy HK, Lynch HT. Lynch syndrome in the 21st century: clinical perspectives. Qjm 2016;109:151-8. [Crossref] [PubMed]
- Green RC, Parfrey PS, Woods MO, et al. Prediction of Lynch syndrome in consecutive patients with colorectal cancer. J Natl Cancer Inst 2009;101:331-40. [Crossref] [PubMed]
- Kastrinos F, Allen JI, Stockwell DH, et al. Development and validation of a colon cancer risk assessment tool for patients undergoing colonoscopy. Am J Gastroenterol 2009;104:1508-18. [Crossref] [PubMed]
- Monzon JG, Cremin C, Armstrong L, et al. Validation of predictive models for germline mutations in DNA mismatch repair genes in colorectal cancer. Int J Cancer 2010;126:930-9. [PubMed]
- Boland CR, Shike M. Report from the Jerusalem workshop on Lynch syndrome-hereditary nonpolyposis colorectal cancer. Gastroenterology 2010;138:2197.e1-7. [Crossref] [PubMed]
- Moreira L, Balaguer F, Lindor N, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA 2012;308:1555-65. [Crossref] [PubMed]
- Shawki S, Kalady MF. Recent advances in understanding Lynch syndrome. F1000Res 2016;5:2889. [Crossref] [PubMed]
- Farchoukh L, Kuan SF, Dudley B, et al. MLH1-deficient Colorectal Carcinoma With Wild-type BRAF and MLH1 Promoter Hypermethylation Harbor KRAS Mutations and Arise From Conventional Adenomas. Am J Surg Pathol 2016;40:1390-9. [Crossref] [PubMed]
- Gupta S, Provenzale D, Regenbogen SE, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 3.2017. J Natl Compr Canc Netw 2017;15:1465-75. [Crossref] [PubMed]
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a Variety of Mutations in Cancer Predisposition Genes in Patients With Suspected Lynch Syndrome. Gastroenterology 2015;149:604-13.e20. [Crossref] [PubMed]
- Plon SE, Eccles DM, Easton D, et al. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat 2008;29:1282-91. [Crossref] [PubMed]
- You YN, Vilar E. Classifying MMR variants: time for revised nomenclature in Lynch syndrome. Clin Cancer Res 2013;19:2280-2. [Crossref] [PubMed]
- Syngal S, Fox EA, Li C, et al. Interpretation of genetic test results for hereditary nonpolyposis colorectal cancer: implications for clinical predisposition testing. JAMA 1999;282:247-53. [Crossref] [PubMed]
- Boland CR. The mystery of mismatch repair deficiency: lynch or lynch-like? Gastroenterology 2013;144:868-70. [Crossref] [PubMed]
- Carethers JM. Differentiating Lynch-like from Lynch syndrome. Gastroenterology 2014;146:602-4. [Crossref] [PubMed]
- Mensenkamp AR, Vogelaar IP, van Zelst-Stams WA, et al. Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in Lynch syndrome-like tumors. Gastroenterology 2014;146:643-6.e8. [Crossref] [PubMed]
- Chika N, Eguchi H, Kumamoto K, et al. Prevalence of Lynch syndrome and Lynch-like syndrome among patients with colorectal cancer in a Japanese hospital-based population. Jpn J Clin Oncol 2017;47:108-17. [PubMed]
- Kim TJ, Kim ER, Hong SN, et al. Survival Outcome and Risk of Metachronous Colorectal Cancer After Surgery in Lynch Syndrome. Ann Surg Oncol 2017;24:1085-92. [Crossref] [PubMed]
- Lynch HT. Is there a role for prophylactic subtotal colectomy among hereditary nonpolyposis colorectal cancer germline mutation carriers? Dis Colon Rectum 1996;39:109-10. [Crossref] [PubMed]
- Kalady MF, McGannon E, Vogel JD, et al. Risk of colorectal adenoma and carcinoma after colectomy for colorectal cancer in patients meeting Amsterdam criteria. Ann Surg 2010;252:507-11; discussion 11-3. [PubMed]
- Renkonen-Sinisalo L, Seppala TT, Jarvinen HJ, et al. Subtotal Colectomy for Colon Cancer Reduces the Need for Subsequent Surgery in Lynch Syndrome. Dis Colon Rectum 2017;60:792-9. [Crossref] [PubMed]
- You YN, Chua HK, Nelson H, et al. Segmental vs. extended colectomy: measurable differences in morbidity, function, and quality of life. Dis Colon Rectum 2008;51:1036-43. [Crossref] [PubMed]
- Kiran RP, El-Gazzaz G, Remzi FH, et al. Influence of age at ileoanal pouch creation on long-term changes in functional outcomes. Colorectal Dis 2011;13:184-90. [Crossref] [PubMed]
- Haanstra JF, de Vos Tot Nederveen Cappel WH, Gopie JP, et al. Quality of life after surgery for colon cancer in patients with Lynch syndrome: partial versus subtotal colectomy. Dis Colon Rectum 2012;55:653-9. [Crossref] [PubMed]
- Meyers M, Wagner MW, Hwang HS, et al. Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine-mediated cell death and cell cycle responses. Cancer Res 2001;61:5193-201. [PubMed]
- Bracht K, Nicholls AM, Liu Y, et al. 5-Fluorouracil response in a large panel of colorectal cancer cell lines is associated with mismatch repair deficiency. Br J Cancer 2010;103:340-6. [Crossref] [PubMed]
- Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219-26. [Crossref] [PubMed]
- Alex AK, Siqueira S, Coudry R, et al. Response to Chemotherapy and Prognosis in Metastatic Colorectal Cancer With DNA Deficient Mismatch Repair. Clin Colorectal Cancer 2017;16:228-39. [Crossref] [PubMed]
- Burn J, Sheth H. The role of aspirin in preventing colorectal cancer. British Medical Bulletin 2016;119:17-24. [Crossref] [PubMed]
- Begum R, Martin SA. Targeting Mismatch Repair defects: A novel strategy for personalized cancer treatment. DNA Repair (Amst) 2016;38:135-9. [Crossref] [PubMed]
- Zhang YZ, Sheng JQ, Li SR, et al. Hereditary predisposition of colorectal cancer and prevalence of hereditary nonpolyposis colorectal cancer in general population of colorectal cancer patients in China. Zhonghua Yi Xue Za Zhi 2005;85:2995-3000. [PubMed]
- Furukawa T, Konishi F, Shitoh K, et al. Evaluation of screening strategy for detecting hereditary nonpolyposis colorectal carcinoma. Cancer 2002;94:911-20. [Crossref] [PubMed]
- Kumamoto K, Ishida H, Suzuki O, et al. Lower prevalence of Lynch syndrome in colorectal cancer patients in a Japanese hospital-based population. Surg Today 2016;46:713-20. [Crossref] [PubMed]
- Shin JS, Tut TG, Yang T, et al. Radiotherapy response in microsatellite instability related rectal cancer. Korean J Pathol 2013;47:1-8. [Crossref] [PubMed]
- Franchitto A, Pichierri P, Piergentili R, et al. The mammalian mismatch repair protein MSH2 is required for correct MRE11 and RAD51 relocalization and for efficient cell cycle arrest induced by ionizing radiation in G2 phase. Oncogene 2003;22:2110-20. [Crossref] [PubMed]
- Martin L, Marples B, Coffey M, et al. Recognition of O6MeG lesions by MGMT and mismatch repair proficiency may be a prerequisite for low-dose radiation hypersensitivity. Radiat Res 2009;172:405-13. [Crossref] [PubMed]
- Charara M, Edmonston TB, Burkholder S, et al. Microsatellite status and cell cycle associated markers in rectal cancer patients undergoing a combined regimen of 5-FU and CPT-11 chemotherapy and radiotherapy. Anticancer Res 2004;24:3161-7. [PubMed]
- Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 2000;118:829-34. [Crossref] [PubMed]
- Jenkins MA, Dowty JG, Ait Ouakrim D, et al. Short-term risk of colorectal cancer in individuals with lynch syndrome: a meta-analysis. J Clin Oncol 2015;33:326-31. [Crossref] [PubMed]
- Edelstein DL, Axilbund J, Baxter M, et al. Rapid development of colorectal neoplasia in patients with Lynch syndrome. Clin Gastroenterol Hepatol 2011;9:340-3. [Crossref] [PubMed]
- Burn J, Bishop DT, Mecklin JP, et al. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N Engl J Med 2008;359:2567-78. [Crossref] [PubMed]
- Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011;378:2081-7. [Crossref] [PubMed]
- Lu JY, Sheng JQ. Advances in the study of Lynch syndrome in China. World J Gastroenterol 2015;21:6861-71. [Crossref] [PubMed]
- Shu Z, Yanqin H, Ying Y. Hereditary colorectal cancer in china. Hered Cancer Clin Pract 2005;3:155-64. [Crossref] [PubMed]
- Wei W, Liu F, Liu L, et al. Distinct mutations in MLH1 and MSH2 genes in hereditary non-polyposis colorectal cancer (HNPCC) families from China. BMB Rep 2011;44:317-22. [Crossref] [PubMed]
- Jin HY, Ding YJ, Liu XF, et al. Screening the hereditary nonpolyposis colorectal cancer by revised Bethesda guideline: a cohort study of 110 cases. Zhonghua Yi Xue Za Zhi 2007;87:1445-7. [PubMed]
- Sugano K, Nakajima T, Sekine S, et al. Germline PMS2 mutation screened by mismatch repair protein immunohistochemistry of colorectal cancer in Japan. Cancer Sci 2016;107:1677-86. [Crossref] [PubMed]
- Cai SJ, Xu Y, Cai GX, et al. Clinical characteristics and diagnosis of patients with hereditary nonpolyposis colorectal cancer. World J Gastroenterol 2003;9:284-7. [Crossref] [PubMed]
- Shen H, Yuan Y, Song YM, et al. Comparison of clinical and genetic phenotypes between Chinese and Korean hereditary nonpolyposis colorectal cancer families. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2008;25:326-30. [PubMed]
- Tanakaya K, Yamaguchi T, Ishikawa H, et al. Causes of Cancer Death Among First-Degree Relatives in Japanese Families with Lynch Syndrome. Anticancer Res 2016;36:1985-9. [PubMed]
- Wang XL, Yuan Y, Zhang SZ, et al. Clinical and genetic characteristics of Chinese hereditary nonpolyposis colorectal cancer families. World J Gastroenterol 2006;12:4074-7. [Crossref] [PubMed]
- Park YJ, Shin KH, Park JG. Risk of gastric cancer in hereditary nonpolyposis colorectal cancer in Korea. Clin Cancer Res 2000;6:2994-8. [PubMed]
- Yamaguchi T, Furukawa Y, Nakamura Y, et al. Comparison of clinical features between suspected familial colorectal cancer type X and Lynch syndrome in Japanese patients with colorectal cancer: a cross-sectional study conducted by the Japanese Society for Cancer of the Colon and Rectum. Jpn J Clin Oncol 2015;45:153-9. [Crossref] [PubMed]
- Chew MH, Koh PK, Ng KH, et al. Phenotypic characteristics of hereditary non-polyposis colorectal cancer by the Amsterdam criteria: an Asian perspective. ANZ J Surg 2008;78:556-60. [Crossref] [PubMed]
- Liu F, Yang L, Zhou X, et al. Clinicopathological and genetic features of Chinese hereditary nonpolyposis colorectal cancer (HNPCC). Med Oncol 2014;31:223. [Crossref] [PubMed]
- Luo DC, Cai Q, Sun MH, et al. Clinicopathological and molecular genetic analysis of HNPCC in China. World J Gastroenterol 2005;11:1673-9. [Crossref] [PubMed]
- Yuan Y, Cao WM, Cai SR, et al. Clinical phenotype of Chinese hereditary nonpolyposis colorectal cancer (HNPCC) families. Zhonghua Zhong Liu Za Zhi 2006;28:36-8. [PubMed]
- Sheng J, Shen Z, Fan C. Clinical phenotypes of hereditary nonpolyposis colorectal cancer in Chinese population. Zhonghua Yi Xue Za Zhi 2002;82:1371-4. [PubMed]
- Yamano T, Hamanaka M, Babaya A, et al. Management strategies in Lynch syndrome and familial adenomatous polyposis: a national healthcare survey in Japan. Cancer Sci 2017;108:243-9. [Crossref] [PubMed]
- Sheng JQ, Li SR, Yang XY, et al. Clinical management of adenomatous polyposis in patients with hereditary non-polyposis colorectal cancer and familial adenomatous polyposis. Zhonghua Yi Xue Za Zhi 2006;86:526-9. [PubMed]


