Sweet syndrome as the leading symptom in the diagnosis of gastric cancer
Introduction
Paraneoplastic syndromes are defined as those which may appear when tumour cell-released substances alter the correct functions of nearby cells or tissues (1). Their incidence is not well defined, although they are more frequent in hematologic cancers and, among solid cancers, in those patients suffering from breast cancer. Moreover, they can appear either at the beginning or during the course of the disease.
We here present an old patient treated for a locally-advanced gastric cancer after the development of a paraneoplastic Sweet syndrome which lead to the final diagnosis.
Case presentation
A 72-year-old patient was admitted to the Internal Medicine Department referring syncopal episodes which were associated with dizziness, diaphoresis, nausea without vomiting, hypotension and temperature up to 39 °C. These were accompanied by macular lesions in forearms, thorax and abdomen. He also reported a weight loss of 9 kilograms in a 2-month time.
As previous medical history, underline he suffered from heart failure NYHA grade III secondary to a heart attack treated with angioplasty and vascular stent, 5 years before the present episode.
At the physical examination, he presented erythematous-oedematous fixed lesions in superior limbs, thorax and abdomen, with an average diameter of 10 mm, and with defined non-confluent oedematous borders (Figure 1). No purpuric nor infiltrative characteristics were present, as well as absence of whitening with diascopy. Other dermatologic or mucosal lesions were not present.
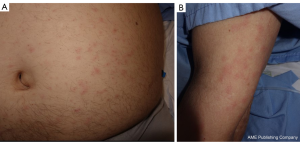
During the study of this episode, several tests were conducted. Blood test revealed leucocytosis (17.8×109/L, normal 3.8×109–10.8×109/L) with neutrophilia (15,400×109/L, normal 1.8×109–7.5×109/L), as well as lymphopenia (950×109/L, normal 1.5×109–4×109/L) and an increased reactive C protein (23.3 mg/dL, normal 0–5 mg/dL)
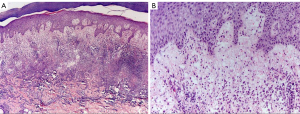
A punch from de affected skin was performed, with the result of interstitial diffuse dermatitis with polymorphonuclear and histiocytic cells and intense oedema, associating CD68 positive in the interstice (Figure 2). All compatible with acute neutrophilic dermatosis.
With the suspicion of this being secondary to a systemic illness, imaging test were conducted. CT scan showed focal thickening of the gastric wall with nearby celiac and retroperitoneal lymphadenopathies as well as subcentimetric pulmonary nodules in inferior right lobe of the lung.
Diagnostic gastroscopy was performed, where an ulcerated vegetative neoplasia at the anterior gastric body with extension to the fundus was biopsied.
After all the study, the patient was diagnosed of a metastatic adenocarcinoma of the stomach poorly differentiated HER2 positive by FISH; with an associated paraneoplastic acute neutrophilic dermatosis (Sweet syndrome).
Treatment with prednisone 30 mg a day was established, with complete response of the lesions after a month of treatment and normalization of the blood parameters (leucocytes 11,000×109/L, neutrophils 8,000×109/L, reactive C protein 2 mg/dL).
During the surveillance of the patient, previous to the beginning of the oncologic treatment, he presented to the Emergency Department referring instability when walking. Brain MRI was performed, noticing a cystic and solid lesion on the left cerebellar hemisphere. Glucocorticoids in combination with holocraneal radiotherapy were given, with good management of symptoms.
However, few days later, que presented with high digestive haemorrhage which required blood transfusions and haemostatic radiotherapy to cope with it.
Finally, after all these complications, patient had very poor performance status and was completely dependent on his caregiver, so best supportive care was chosen as the more suitable management plan.
At the time of the writing of this report, the patient was living at home with his caregiver with a good quality of life and has had no recurrence of his Sweet syndrome nor any other paraneoplastic disorder.
Discussion
Acute neutrophilic dermatosis or Sweet syndrome (2) often debuts as a papular rash or as indurated and painful erythematous plaques in face, limbs and thorax. Paraneoplastic Sweet syndrome accounts for the 20% of all the cases. From these, 85% appear accompanying haematological cancers (mostly acute myeloid leukaemia), and the other 15% associated with solid tumours, such adenocarcinomas from the breast, upper genitourinary tract and upper intestinal tract (3).
Its pathogeny is by the moment not completely understood. Nevertheless, at least three factors have been proposed to trigger it: hypersensitivity reaction to cancer neoantigens and cytokine production (4); cytokine dysregulation such as granulocyte-colony stimulating factor (G-CSF) production increasing circulating neutrophils in peripheral blood (5); and genetic susceptibility, such as alterations in chromosome 3q (6).
Clinical manifestations include fever, poor general condition and joint and muscular pain, apart from the typical dermic lesions described before. In nearly 50% of the cases extra cutaneous disease can be present, affecting the liver, the kidneys or the lungs (as a reticular or nodular infiltration with pleural effusion) (7,8).
Blood test show increased glomerular sedimentation speed and reactive C protein parameters, as well as leucocytosis with neutrophils accounting for more than the 70% of them.
Under the microscope, a dense interstitial and perivascular neutrophilic infiltration in the papillary dermis is characteristic, with oedema and nuclear fragmentation of mature neutrophils, without associated vasculitis (9).
Definitive diagnosis of paraneoplastic syndrome must be made according to the modified Su-Liu criteria (7), which include major criteria (sudden appearance of painful erythematous plaque or nodule and dense neutrophilic dermic infiltration without vasculitis in the biopsy of a lesion) and minor criteria (fever >38 °C, glomerular sedimentation speed >20 mm/h, leucocytes >8×109/L, neutrophils >70%, high reactive C protein, good response to glucocorticoid therapy and history of gastrointestinal infection, neoplasia or recent immunization).
Differential diagnosis should include infectious disorders, as bacterial sepsis may induce similar symptoms and dermic affectation; and other dermatologic syndromes, such as other neutrophilic dermatoses (i.e., pyoderma gangrenosum or Behçet syndrome) or medium-vessel vasculitis, among others (8).
Treatment of Sweet syndrome is based on glucocorticoids, with a starting dose of prednisone 30 mg, followed by a progressive descendent dosing being the most recommended in the literature (7). If this may not work, second line treatments may be used, such as indomethacin, cyclosporine or dapsone, among others (8).
The majority of cases will respond satisfactorily to the treatment, although up to 20–30% of recurrences have been reported. These recurrences may be indicative of disease progression (8).
In conclusion, our patient presented prior to cancer diagnosis with a paraneoplastic Sweet syndrome, with complete response to glucocorticoid treatment. Hence, a recommendation of a complete study suspecting a systemic disease, mainly cancer, can be made when this or other related syndromes related to cancer appear, so that de diagnosis is not unnoticed and the prognosis of the patient may be improved.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The patient and caregivers involved gave consent to publication of this manuscript and any accompanying images.
References
- Gethi.org. Madrid: Grupo Español Tumores Huérfanos e Infrecuentes (GETHI). Available online: http://www.gethi.org/Portals/0/libro_digital_oncologia-V2.pdf
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol 1964;76:349-56. [Crossref] [PubMed]
- Cohen PR, Kurzrock R. Sweet's syndrome and cancer and cancer. Clin Dermatol 1993;11:149-57. [Crossref] [PubMed]
- Voelter-Mahlknecht S, Bauer J, Metzler G, et al. Bullous variant of Sweet's syndrome. Int J Dermatol 2005;44:946-7. [Crossref] [PubMed]
- Shinojima Y, Toma Y, Terui T. Sweet syndrome associated with intrahepatic cholangiocarcinoma producing granulocyte colony-stimulating factor. Br J Dermatol 2006;155:1103-4. [Crossref] [PubMed]
- Mijovic A, Novak A, Medenica L. Sweet's syndrome associated with inversion of chromosome 3q in a patient with refractory anemia. Eur J Haematol 1992;49:156-7. [Crossref] [PubMed]
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet Syndrome: A Review and Update. Actas Dermosifiliogr 2016;107:369-78. [Crossref] [PubMed]
- Paydas S. Sweet's syndrome: a revisit for hematologists and oncologists. Crit Rev Oncol Hematol 2013;86:85-95. [Crossref] [PubMed]
- Watanabe T, Nakashima K, Shindo M, et al. Multiorgan involvement in Sweet's syndrome. Clin Exp Dermatol 2009;34:e343-4. [Crossref] [PubMed]


