Role of immune checkpoint inhibitors and novel immunotherapies in uveal melanoma
Introduction of uveal melanoma (UM)
The eye is organ of sight. It is globe like structure which is mostly filled with a gel like substance called vitreous. The main anatomical component of eye includes cornea, sclera, uvea and retina. Cornea is anterior most part of eye and it helps in focusing light on retina with the help of pupil and lens. Uvea consist of three parts choroid, ciliary body and Iris. The main function of uvea is to provide nutrition and gas exchange; it is highly vascular structure. Retina is the sensory screen which converts light into electric signal and transmit it to brain with the help of optic nerve which is seen in eye as optic disc. Uvea consist of endothelial cells, melanocytes and immune cells. Melanocytes are the cells which provide color to the eye, and are the cells from where UM originates.
UM is rare disease and constitutes 0.1% of all cancer deaths. The incidence of UM is approximately six cases per million per year in USA (1). It represents around 3–5% of all melanomas (2,3). Although it is considered as a rare tumor but it is most common intraocular tumor in adults and it constitutes 85–90% of all ocular melanoma cases (4). Uveal tract consists of three components iris, Ciliary body and choroid and all contains melanocytes which has potential to undergo transformation to form UM. Choroid is the most common site for UM and 85% of UMs arises from choroid. Rest of them arise from ciliary body (5–8%) and iris (3–5%) (5). There are several risk factors which are associated with UM like ultraviolet radiation exposure, fair skin, light eye color and presence of nevi (6). UM is a tumor of old age as it is generally diagnosed between 60-70 years of age (7,8). The incidence of UM is more in males as compared to females (4).
There are three main objectives of treatment: save life, save vision and save eye. Currently main modalities of treatment are radiotherapy and surgery. Despite these therapies chances of metastasis is quite high in UM. Around 50% of patients develop metastasis within 15 years of diagnosis (9). The overall survival (OS) rate is around 60% in first 5 years and major cause of mortality is metastasis (10-13). After developing liver metastasis mean survival is reduced to 6 months and death rate of 80% at 1 year (14). The most common sites of metastasis are liver, lung and soft tissue and bone (15).
Genetics and pathways involved in UM
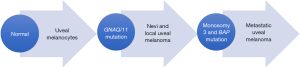
Pathophysiology of cutaneous melanoma and UM are not similar. Mutations in cutaneous melanoma are generally activating mutation in BRAF (52%), RAS (10–25%) or loss of function in NF1 gene (16-17). These mutations are not there in UM. Generally, UM have low level of genomic instability and aneuploidy in comparison with other cancer types. In two different studies with total 180 UM patients, 66% of them were diploid and 33% have some aneuploidy (18,19). The commonest abnormalities were loss on 1p, 3, 6q, 9p and gain on 1q, 6p and 8q (20,21).
GNAQ/GNA11 gene mutations
Most of the UM have shown activation of MAPK (mitogen activating protein kinase) pathways suggesting there is upstream mutation (22,23). KIT and RAS family gene mutations can activate MAPK pathway, but they were not present in UM (24,25). For a long time, it has been unknown, which mutation is causing MAPK activation, until the discovery of mutation in GNAQ and GNA11 (encodes Gaq subunit). GNAQ/GNA11 mutations are commonly seen in uveal nevi and UM regardless of cytogenetic status (26,27). But these mutations don’t have potential for full malignant transformations to melanoma rather these mutations are an initiating event for development of UM (28). Along with activation of MAPK, mutation in GNAQ/11 also activates YAP1 (Yes associated protein 1), PKC, AKT. In some of the tumor models YAP1 is associated with tumor growth (29,30).
Monosomy 3
Loss of one copy of chromosome 3 is most significant marker of UM. It is associated with metastasis, poor clinical and histopathological prognosis (31,32). Chromosome 3 harbor tumor suppressor gene for UM progression (33). It has been shown mutation in BAP (BRCA associated protein) on chromosome 3 along with monosomy of chromosome 3 lead to malignant transformation of UM (Figure 1). Mutation in BAP occurred only in metastasizing UM that already lost the other gene on chromosome 3 (34). This constitutes double hit recessive gene mutation.
Ophthalmic immune privileges
Eye is an extremely delicate organ which serves function of vision by an extremely fine balance of optical physics, neuronal electric circuits and its close connection with brain. Slight inflammation in eye can lead to deleterious effects and vision loss. To prevent this, eye attempts to decrease the local and systemic inflammatory responses in eye. This process is known as immune privilege (35). By various mechanisms eye maintains its immune privilege nature some of them are physical barriers like blood retina barrier and absence of efferent lymphatics, these barriers prevent movement of antigens in and out of eye. Ocular microenvironment consists of various immunosuppressive factors which inhibit the inflammatory reaction and immune response like transforming growth factor-beta (TGF-β), complementary regulatory protein (CRP), vasoactive intestinal peptide (VIP), alpha melanocyte stimulating hormone (a-MSH), low expression of MHC, presence of neuropeptides and expression of FAS ligand (36-39). Inhibition of complement activation by increasing the surface molecule expression like CD59, decay accelerating factor (DAF) and membrane cofactor protein also decreases immune response. Along with environment protection, Anterior chamber associated immune deviation (ACAID) is another mechanism by which eye evades immune response. Injection of antigen into anterior chamber (AC) will induce suppression of complement fixing IgG antibody and cell mediated response. After injection, there is release of TNF-α and monocyte chemoattractant protein 1 (MCP-1) in aqueous humor and infiltration of monocytes in Iris. These monocytes migrate into spleen and thymus and activate regulatory T cells which inhibit the cell mediated immune response (40-43). Therefore, ACAID prevents the eye from collateral damage by inflammation along with infection. Recently, program death ligand-1 (PD-L1) and program death ligand-2 (PD-L2) receptors expression has been discovered on retinal pigment epithelial (RPE cells) (44,45). PDL-L1 binds to programmed death receptor 1 (PD-1) and inhibit the immune response. It acts as brakes for activation and proliferation of T cells and therefore provide immune privilege to eye. In some of the preclinical studies it has been shown there is upregulation of PD-L1 by interferon gamma in UM which downregulates immune response and T cell activation.
Immune checkpoint inhibitors (ICIs)
This landmark discovery in management of advanced cancers is based on basic principles of immunology and physiology. When Antigen presenting cells (dendritic cell) activates T cells to fight against cancer cells (foreign antigens), there are costimulatory molecules like CD28 and B-7, their interaction enhances the activation of T cells. But this immune response need to be controlled process, to prevent unchecked activation of T cells and exaggerated immune reactions. For this nature has deployed brakes in form of immune checkpoints like CTLA-4 and PD-1. CTLA4 has higher affinity for B-7 and it negatively regulate the immune response. Similarly, PD-1 binds with PD-L1 and downregulates the immune response. So basically, immune checkpoint act as brakes of immune response (46,47). Cancer cells misuse these receptors and upregulate them, to suppress immune response and promote cancer cell proliferation. ICIs are antibodies which bind to checkpoints and suppresses them therefore, lead to activations and proliferation of T cells which helps in lysis and degradation of cancer cells (48,49).
Rationale for use of ICIs in UM
ICIs revolutionized the treatment of metastatic cancer, Malignant melanoma was the first malignancy for which it was FDA approved. But in almost all the studies of cutaneous melanoma, UM was excluded. As cutaneous melanoma and UM belong to same group of cancers there are chances that ICIs are helpful in management of malignant UM too. UM is known for its long latency period with late metastatic recurrence after initial treatment. This long period of dormancy indicates that there is some immunologic control which slows the tumor spread. But once tumor is able to overcome this immunologic control, it can spread to different organs of body especially liver and have extremely fatal outcome with OS of roughly 6 months to 1 year. In some of the preclinical studies, it has been shown that tumor dormancy is related to CD8+ T cell (50). So long latency period can be due to immunologic status of UM. Microenvironment of metastatic UM is less studied to understand the immunological status. A study was conducted to compare the tumor infiltrating lymphocytes (TIL) activity in liver metastases in patients of UM and cutaneous melanoma. Total 16 UM patients and 35 cutaneous melanoma patients were enrolled. TIL activity was much more in cutaneous melanoma patients, but a subset of UM patients showed comparable TIL activity as compared to cutaneous melanoma.
In contrast to what has been shown in other type of cancers, lymphocytic cell infiltration is associated with poor prognosis in primary UM (51). In one of the largest UM studies (52), total 1,078 patients were evaluated and out of that 134 (12.4%) had high lymphocytic infiltrate. Even after controlling for other risk factors, this was associated with poor survival. It is not clear why prognosis is poor in patients with high TIL in primary UM. Tumor associated macrophages are also poor prognostic markers for primary UM. In a study of it has been shown that primary UM has been infiltrated with macrophages of M2 phenotype and this phenotype promote tumor growth and has angiogenic properties (53,54). This macrophage infiltration is also associated with monosomy of chromosome 3, which may be the reason for bad prognosis (53).
Development of autoimmune phenomena is associated with better outcome in patients with advanced UM. In a study among UM patients it has shown than around 13.2% had autoimmune hypothyroidism and 8.8% had other systemic autoimmune disease (55). Patients with some kind of autoimmune disease had trend toward better survival (55). Therefore, suggesting that systemic autoimmunity has some role in changing the activity of metastatic UM.
ICIs in UM
Anti CTLA-4 antibodies
Ipilimumab is an ICI, which binds and blocks CTLA-4 receptor. It is humanized monoclonal antibody, and was first approved for management of advanced melanoma that has showed progression on at least one prior therapy. But most of the trials of cutaneous melanoma did not include UM patients. But several small studies have reported the efficacy of ipilimumab in UM which are summarized in Table 1.

Full table
Zimmer et al. (56) in phase II DeCOG study of ipilimumab in metastatic UM patients have shown, that median OS was 6.8 months and median progression-free survival (PFS) was 2.8 months. The disease control rate (DCR) was 47% and 21% at 12 and 24 weeks respectively. No one experienced complete or partial response, but 16 patients had stable disease and 36% patients had grade 3–4 adverse events. Maio et al. (57) did a study on safety and efficacy of ipilimumab in advanced UM. They assessed 82 patients, at a median follow-up of 5.6 months, they have shown a OS and PFS of 6 and 3.6 months respectively. At 1-year OS and PFS was 31% and 11% and safely was similar to patients with cutaneous melanoma. Kelderman et al. (58) studied role of Ipilimumab in unresectable and metastatic UM and who have received one prior treatment for metastatic disease. Total 22 patients were enrolled in study and ipilimumab was given at 1, 4, 7, 10 weeks. The OS and PFS was 5.2 and 2.9 months respectively. Out of 22, 13 patients showed disease progression, 1 had partial response and no one had complete response. Luke et al. (61) did a multicenter retrospective analysis of four hospitals from Europe and United States. Total 39 patients were analyzed and out of them 34 received Ipilimumab at 3 mg/kg and 5 at 10 mg/kg. The response rate was 2.6% at 12 and 23 weeks. Immune-related adverse events (irAE) were more common in 10-mg/kg group than 3-mg/kg group. The OS was 9.6 months and survival was significantly associated with performance status, Absolute lymphocyte count and LDH levels. Deo et al. (63) also did a retrospective analysis, to study long-term survival benefit from ipilimumab in previously treated malignant UM patients. Out of total 24 patients, no objective response was seen in 96% patients (23), only one showed partial response. Median OS and PFS was 9.7 and 2.8 months respectively. OS rates at 12 and 24 months were 45.6% and 11.4%. This study showed low response rate but there was OS benefit in some patients.
Two studies were done with Ipilimumab dose of 10 mg/kg in UM. One of them conducted by Piulats et al. (60) which is an open label, phase II, single arm trial to evaluate Ipilimumab in Metastatic UM patients. It was conducted in five centers in Spain and 32 patients were enrolled. Induction therapy consist of 4 cycles of Ipilimumab at dose of 10 mg/kg Q3W, and maintenance therapy every Q12W. The median OS was 9.8 months and partial response was seen in 6.5% of patients. Grade 3–4 adverse events were reported as 10%. Second one was done by Danielli et al. (59) as a sub-analysis to assess the Ipilimumab safety and activity in UM patients. Total 13 patients were treated at six European centers. OS was 9 months, no objective response was observed, 23% (three patients) had grade 3 irAE.
Joshua et al. (66) conducted an open label, phase II, multicentric study of tremelimumab on patients with advanced melanoma who never got prior immunotherapy. tremelimumab is another CTLA-4 inhibitor like Ipilimumab. Patients got up to 4 cycles of tremelimumab at 15 mg/kg dose given every 3 months. Total 11 patients were enrolled in study. The median OS and PFS was 12.8 and 2.9 months respectively. PFS at 6 months was 9.1%. The study was stopped at the first interim stage due to lack of response and modest PFS.
Therefore, CTLA-4 inhibitors have limited activity in metastatic UM. The overall response rate of ipilimumab was around 0–5% at dose of 3 mg/kg, 0–6.5% at dose of 10 mg/kg and PFS was around 3 months. Tremelimumab study was stopped in between due to inadequate response. The side effect profile was similar to studies of CTLA-4 inhibitors in cutaneous melanoma. So, the above-mentioned results do not support the individual use of CTLA-4 inhibitors in advanced UM patients.
Anti PD-1/PD-L1 antibodies
Nivolumab and Pembrolizumab are the two Anti PD-1 antibodies which inhibit the interaction between PD-1 and PD-L1, activates the immune system and leads to lysis of tumor cells. They have been approved by FDA for metastatic cutaneous melanoma and have shown better efficacy and side effect profile as compared to ipilimumab (76-78). But in most of the trials, UM patients were not included. In Table 1, we have summarized the studies regarding ICIs in UM.
Schadendorf et al. (73) conducted a phase II, single arm, open label, multicenter study regarding efficacy of Nivolumab in patients of UM who had disease progression on ipilimumab. In this study, nivolumab was given at dose of 3 mg/kg Q2W for up to 2 years until disease progression or unacceptable toxicity. This study showed median OS of 11 months and two patients had partial response and 15 patients (44%) had disease stabilization at 1 year. OS rate at 1 year was 47% but there was no data on PFS and side effects.
Algazi et al. (69) did a retrospective analysis of role of PD-1/PD-L1 antibodies in UM at nine centers. Total 56 patients were eligible and out of them 38 (68%) received pembrolizumab, 16 (29%) received nivolumab and 2 (4%) received atezolizumab. It showed overall response rate of 3.6%, stable disease in 9% (five patients), OS was 7.6 months and PFS was 2.6 months. Only one patient was removed from the study due to side effects, otherwise treatment was well tolerated. Karydis et al. (72) did a retrospective study on efficacy and safety of pembrolizumab at two centers. Total 25 patients were enrolled who received pembrolizumab at dose of 2 mg/kg Q3W. Results of this study showed the PFS of 3 months and OS was not reached. The outcomes were better in patients with extrahepatic disease as compared to patients who had only liver involvement. Twenty-five percent patients had grade 3–4 adverse events with no treatment related death. As this trial showed better outcome in patient with extrahepatic disease, pembrolizumab should be tried with liver targeted therapies. Bender et al. (68) did a retrospective study on 15 patients of UM, at two German hospitals. They received PD-1 antibody at dosing schedule of 2 mg/kg Q3W of pembrolizumab or 3 mg/kg Q2W of nivolumab. Out of 15, 4 patients received nivolumab and 11 patients got pembrolizumab. This study showed PFS and OS of 3 and 5 months respectively. Stable disease in four patients was best response. In most of patients, disease control was not long lasting and disease progression was seen within 24 weeks with hepatic metastasis. Patients with elevated serum LDH and multiple organ metastasis had poorer response.
Combination of ICI’s
Heppt et al. (67) did a combination study of CTLA-4 inhibitor and PD-1 inhibitor. Patients with metastatic UM were treated with PD-1 inhibitor alone or combination of ipilimumab and PD-1 Inhibitor. Total 96 patients from 20 different German centers were included in the study. Eighty-six patients were treated with PD-1 inhibitors, out of which 54 with pembrolizumab and 32 with nivolumab. Fifteen patients got combination therapy. In individual therapy group OS was 14 and 10 months in pembrolizumab and nivolumab treated patients respectively. In combination therapy group, two patients had partial response and median OS was not reached. Elevated LDH, CRP and relative eosinophil count of <1.5% are independent factors for poor prognosis. So, this study showed poor outcome with even combination therapy of ICIs.
So similar to Anti CTLA-4 antibody, anti PD-1 and Anti PD-L1 antibodies were unable to show significant benefit in patients with metastatic UM. Some of the retrospective studies showed occasional response but overall response and clinical benefit was not up to the mark. So individual CTLA-4 or PD-1/PD-L1 antibodies were not successful in metastatic UM, but there are only few studies regarding combination of ICIs. Therefore, more clinical trials are needed to completely understand the efficacy of combinational therapy of ICIs in UM. Some of the active trials are mentioned in Table 2.

Full table
Other immunotherapies in UM
UM has low mutational burden and low PD-L1 expression which are the major reason for failure of ICIs in UM. Despite their failure, recent research on dendritic vaccine against antigen gp100 have shown promising results. Bol et al. (79) conducted a study to know efficacy and safety of dendritic cell vaccine in metastatic UM patients. Total 14 patients were treated with at least three vaccinations with autologous dendritic cells (antigen presenting cells with antigen gp100 and tyrosinase). The median OS was 19.2 months and four patients (29%) had tumor specific immune response.
MAA (melanoma associated antigens) are extremely immunogenic antigens and they are identified by T cells. Both types of melanoma have wide variety of MAA, some of them are Melan-A/MART-1 (80,81), gp100 (82) and tyrosinase (83,84) (Tyr). gp100 is a differentiation antigen which is present on melanocytes and overexpressed in both UM and cutaneous melanoma cells (85), but UM is more consistent in expression of this antigen at high levels.
The main reason for cancer progression and proliferation is inability of immune system to detect cancer cells. Cancer antigens are not detected by T cells so there is no immune response against these antigens. IMCgp100 is answer to this problem as it is an engineered T-cell receptor (TCR) which is a bispecific, and act a bridge between antigen and cytotoxic T cells. These engineered TCR are specific for protein gp100 which is expressed on ocular melanoma cells. It has two functional ends one is targeting end which has enhanced affinity to bind to gp100 antigen and other is effector end which is TCR fused to an anti CD3 antibody single chain variable fragment (scFv). Basically, the target end bind with the antigen (gp100) with high affinity and effector end with it anti CD-3 scFv, therefore leads to activation of cytotoxic T cells. Activation of T cells leads to lysis and degradation of cancer cells.
In ASCO 2016 annual meeting, results of first in human study of IMCgp100 was presented (86). It is a phase I, open label, dose finding study to assess tolerability and efficacy of IMCgp100. Total 84 patients with metastatic melanoma were enrolled in this study and 16 out of them had UM. Other than infusion related reactions, adverse events seen with weekly dosing are grade 1 and 2. In dose escalating group, most common adverse events are rash, pruritus, pyrexia and periorbital edema. Hypotension was seen during the first dose of IMCgp100 but that was likely due to movement of T lymphocytes and chemokine release in tumor microenvironment. At society for melanoma research congress 2016, results of UM cohort of patients were presented (87). In this study, seven patients (47%) had stable disease and three patients (20%) achieved partial response. DCR was 53% and 40% at 16 and 24 weeks respectively. After getting initial treatment dose, tumor specimens were obtained on day 2, which showed increase in T cell infiltration. After promising results of initial studies, further studies are ongoing regarding IMCgp100 in UM which are summarized in Table 2.
As we mentioned earlier, the molecular biomarkers of UM are gains and losses of chromosomes with monosomy of 3rd chromosome as a strong indicator for metastasis and poor prognosis. Gene expression profiling (GEP) divided UM into two basic subtypes class 1 and class 2. Class 1 constitutes around two-third of tumors and carries good prognosis and class 2 is associated with poor prognosis. Recently, a new biomarker has been discovered that enhances the GEP accuracy, i.e., PRAME, a cancer testis antigen which is preferentially expressed in melanoma (88,89). PRAME along with a novel biomarker can be a target for immunotherapy as it is not expressed on normal cells. Monoclonal antibody (90) and cytotoxic T lymphocytes (91) against PRAME are new therapeutic options which are under research and they can be new tools in our armamentarium to fight against UM.
Conclusions
After the dramatic success of ICIs in metastatic cutaneous melanoma, UM was next. Till now, ICIs are unable to prove their worth in UM, likely due to lack of PD-L1 receptor and low mutational burden. Individually CTLA-4 or PD-1/PD-L1 inhibitor didn’t show significant clinical response but results of combinational studies are still awaited. Novel immunotherapies like IMCgp100 have shown promising results in early phase studies and new biomarkers like PRAME will be a good tool to determine susceptible patients for immunotherapy. Further multicenter randomized controlled trials are needed to completely understand the role of immunotherapy in UM.
Acknowledgements
Special thanks to Dr. Manisha Dhananjaya.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Osterlind A. Trends in incidence of ocular malignant melanoma in Denmark 1943-1982. Int J Cancer 1987;40:161-4. [Crossref] [PubMed]
- Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology 2011;118:1881-5. [Crossref] [PubMed]
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998;83:1664-78. [Crossref] [PubMed]
- McLaughlin CC, Wu XC, Jemal A, et al. Incidence of noncutaneous melanomas in the U.S. Cancer 2005;103:1000-7. [Crossref] [PubMed]
- Damato B. Progress in the management of patients with uveal melanoma. The 2012 Ashton Lecture. Eye (Lond) 2012;26:1157-72. [Crossref] [PubMed]
- Weis E, Shah CP, Lajous M, et al. The association of cutaneous and iris nevi with uveal melanoma: a meta-analysis. Ophthalmology 2009;116:536-43.e2. [Crossref] [PubMed]
- Damato EM, Damato BE. Detection and time to treatment of uveal melanoma in the United Kingdom: an evaluation of 2,384 patients. Ophthalmology 2012;119:1582-9. [Crossref] [PubMed]
- Andreoli MT, Mieler WF, Leiderman YI. Epidemiological trends in uveal melanoma. Br J Ophthalmol 2015;99:1550-3. [Crossref] [PubMed]
- Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci 2003;44:4651-9. [Crossref] [PubMed]
- Caminal JM, Ribes J, Cleries R, et al. Relative survival of patients with uveal melanoma managed in a single center. Melanoma Res 2012;22:271-7. [Crossref] [PubMed]
- Bergman L, Seregard S, Nilsson B, et al. Uveal melanoma survival in Sweden from 1960 to 1998. Invest Ophthalmol Vis Sci 2003;44:3282-7. [Crossref] [PubMed]
- Singh AD, Topham A. Survival rates with uveal melanoma in the United States: 1973-1997. Ophthalmology 2003;110:962-5. [Crossref] [PubMed]
- Burr JM, Mitry E, Rachet B, et al. Survival from uveal melanoma in England and Wales 1986 to 2001. Ophthalmic Epidemiol 2007;14:3-8. [Crossref] [PubMed]
- Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group report no. Arch Ophthalmol 2005;123:1639-43. [Crossref] [PubMed]
- Rietschel P, Panageas KS, Hanlon C, et al. Variates of survival in metastatic uveal melanoma. J Clin Oncol 2005;23:8076-80. [Crossref] [PubMed]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [Crossref] [PubMed]
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 2015;161:1681-96. [Crossref] [PubMed]
- Coleman K, Baak JP, Van Diest PJ, et al. DNA ploidy status in 84 ocular melanomas: a study of DNA quantitation in ocular melanomas by flow cytometry and automatic and interactive static image analysis. Hum Pathol 1995;26:99-105. [Crossref] [PubMed]
- Karlsson M, Boeryd B, Carstensen J, et al. DNA ploidy and S-phase fraction as prognostic factors in patients with uveal melanomas. Br J Cancer 1995;71:177-81. [Crossref] [PubMed]
- Damato B, Dopierala JA, Coupland SE. Genotypic profiling of 452 choroidal melanomas with multiplex ligation-dependent probe amplification. Clin Cancer Res 2010;16:6083-92. [Crossref] [PubMed]
- Onken MD, Worley LA, Tuscan MD, et al. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J Mol Diagn 2010;12:461-8. [Crossref] [PubMed]
- Weber A, Hengge UR, Urbanik D, et al. Absence of mutations of the BRAF gene and constitutive activation of extracellular-regulated kinase in malignant melanomas of the uvea. Lab Invest 2003;83:1771-6. [Crossref] [PubMed]
- Zuidervaart W, Van Nieuwpoort F, Stark M, et al. Activation of the MAPK pathway is a common event in uveal melanomas although it rarely occurs through mutation of BRAF or RAS. Br J Cancer 2005;92:2032-8. [Crossref] [PubMed]
- Pache M, Glatz K, Bosch D, et al. Sequence analysis and high-throughput immunohistochemical profiling of KIT (CD 117) expression in uveal melanoma using tissue microarrays. Virchows Arch 2003;443:741-4. [Crossref] [PubMed]
- Soparker CN, O’brien JM, Albert DM. Investigation of the role of the ras protooncogene point mutation in human uveal melanomas. Invest Ophthalmol Vis Sci 1993;34:2203-9. [PubMed]
- Bauer J, Kilic E, Vaarwater J, et al. Oncogenic GNAQ mutations are not correlated with disease-free survival in uveal melanoma. Br J Cancer 2009;101:813-5. [Crossref] [PubMed]
- Jensen DE, Rauscher FJ 3rd. BAP1, a candidate tumor suppressor protein that interacts with BRCA1. Ann N Y Acad Sci 1999;886:191-4. [Crossref] [PubMed]
- Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2009;457:599-602. [Crossref] [PubMed]
- Feng X, Degese MS, Iglesias-Bartolome R, et al. Hippo independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell 2014;25:831-45. [Crossref] [PubMed]
- Vaqué JP, Dorsam RT, Feng X, et al. A genome-wide RNAi screen reveals a Trio-regulated Rho GTPase circuitry transducing mitogenic signals initiated by G protein-coupled receptors. Mol Cell 2013;49:94-108. [Crossref] [PubMed]
- Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet 1996;347:1222-5. [Crossref] [PubMed]
- Scholes AG, Damato BE, Nunn J, et al. Monosomy 3 in uveal melanoma: correlation with clinical and histologic predictors of survival. Invest Ophthalmol Vis Sci 2003;44:1008-11. [Crossref] [PubMed]
- Horsthemke B, Prescher G, Bornfeld N, et al. Loss of chromosome 3 alleles and multiplication of chromosome 8 alleles in uveal melanoma. Genes Chromosom Cancer 1992;4:217-21. [Crossref] [PubMed]
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010;330:1410-3. [Crossref] [PubMed]
- Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol 1948;29:58-69. [PubMed]
- Streilein JW, Ksander BR, Taylor AW. Immune deviation in relation to ocular immune privilege. J Immunol 1997;158:3557-60. [PubMed]
- Streilein JW, Takeuchi M, Taylor AW. Immune privilege, T-cell tolerance, and tissue-restricted autoimmunity. Hum Immunol 1997;52:138-43. [Crossref] [PubMed]
- Ferguson TA, Griffith TS. A vision of cell death: insights into immune privilege. Immunol Rev 1997;156:167-84. [Crossref] [PubMed]
- Griffith TS, Brunner T, Fletcher SM, et al. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 1995;270:1189-92. [Crossref] [PubMed]
- Niederkorn JY. Immune privilege in the anterior chamber of the eye. Crit Rev Immunol 2002;22:13-46. [PubMed]
- Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nature Immunology 2006;7:354-9. [Crossref] [PubMed]
- Stein-Streilein J, Streilein JW. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int Rev Immunol 2002;21:123-52. [Crossref] [PubMed]
- Cone RE, Chattopadhyay S, O’Rourke J. Control of delayed-type hypersensitivity by ocular-induced CD8+ regulatory T cells. Chem Immunol Allergy 2008;94:138-49. [Crossref] [PubMed]
- Usui Y, Okunuki Y, Hattori T, et al. Functional expression of B7H1 on retinal pigment epithelial cells. Exp Eye Res 2008;86:52-9. [Crossref] [PubMed]
- Sugita S, Usui Y, Horie S, et al. T-cell suppression by programmed cell death 1 ligand 1 on retinal pigment epithelium during inflammatory conditions. Invest Ophthalmol Vis Sci 2009;50:2862-70. [Crossref] [PubMed]
- Alme AK, Karir BS, Faltas BM, et al. Blocking immune checkpoints in prostate, kidney, and urothelial cancer: an overview. Urol Oncol 2016;34:171-81. [Crossref] [PubMed]
- Goswami S, Aparicio A, Subudhi SK. Immune checkpoint therapies in prostate cancer. Cancer J 2016;22:117-20. [Crossref] [PubMed]
- Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994;1:405-13. [Crossref] [PubMed]
- Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 2013;1:32-42. [Crossref] [PubMed]
- Eyles J, Puaux AL, Wang X, et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest 2010;120:2030-9. [Crossref] [PubMed]
- Rothermel LD, Sabesan AC, Stephens DJ, et al. Identification of an immunogenic subset of metastatic uveal melanoma. Clin Cancer Res 2016;22:2237-49. [Crossref] [PubMed]
- de la Cruz PO Jr, Specht CS. IW ML. Lymphocytic infiltration in uveal malignant melanoma. Cancer 1990;65:112-5. [Crossref] [PubMed]
- Bronkhorst IH, Ly LV, Jordanova ES, et al. Detection of M2-macrophages in uveal melanoma and relation with survival. Invest Ophthalmol Vis Sci 2011;52:643-50. [Crossref] [PubMed]
- Jager MJ, Ly LV, El Filali M, et al. Macrophages in uveal melanoma and in experimental ocular tumor models: friends or foes? Prog Retin Eye Res 2011;30:129-46. [Crossref] [PubMed]
- Ellerhorst JA, Cooksley CD, Grimm EA. Autoimmunity and hypothyroidism in patients with uveal melanoma. Melanoma Res 2001;11:633-7. [Crossref] [PubMed]
- Zimmer L, Vaubel J, Mohr P, et al. Phase II DeCOG-study of ipilimumab in pretreated and treatmentnaive patients with metastatic uveal melanoma. PLoS One 2015;10:e0118564. [Crossref] [PubMed]
- Maio M, Danielli R, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab in patients with pre-treated, uveal melanoma. Ann Oncol 2013;24:2911-5. [Crossref] [PubMed]
- Kelderman S, van der Kooij MK, van den Eertwegh AJ, et al. Ipilimumab in pretreated metastastic uveal melanoma patients. Results of the Dutch Working group on Immunotherapy of Oncology (WIN-O). Acta Oncol 2013;52:1786-8. [Crossref] [PubMed]
- Danielli R, Ridolfi R, Chiarion-Sileni V, et al. Ipilimumab in pretreated patients with metastatic uveal melanoma: safety and clinical efficacy. Cancer Immunol Immunother 2012;61:41-8. [Crossref] [PubMed]
- Piulats Rodriguez JM, Ochoa de Olza M, Codes M, et al. Phase II study evaluating ipilimumab as a single agent in the first-line treatment of adult patients (Pts) with metastatic uveal melanoma (MUM): The GEM-1 trial. J Clin Oncol 2014;32:9033.
- Luke JJ, Callahan MK, Postow MA, et al. Clinical activity of ipilimumab for metastatic uveal melanoma: a retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer 2013;119:3687-95. [Crossref] [PubMed]
- Khattak MA, Fisher R, Hughes P, et al. Ipilimumab activity in advanced uveal melanoma. Melanoma Res 2013;23:79-81. [Crossref] [PubMed]
- Deo MA. Long-term survival benefit from ipilimumab treatment in metastatic uveal melanoma patients. J Clin Oncol 2014;32:3060.
- Shaw H, Larkin J, Corrie P, et al. Ipilimumab for advanced melanoma in an expanded access programme (EAP): ocular, mucosal and acral subtype UK experience. Ann Oncol 2012;23:374. [PubMed]
- Jung M, Lee J, Kim TM, et al. Ipilimumab real-world efficacy and safety in korean melanoma patients from the korean namedpatient program cohort. Cancer Res Treat 2017;49:44-53. [Crossref] [PubMed]
- Joshua AM, Monzon JG, Mihalcioiu C, et al. A phase 2 study of tremelimumab in patients with advanced uveal melanoma. Melanoma Res 2015;25:342-7. [Crossref] [PubMed]
- Heppt MV, Heinzerling L, Kähler KC, et al. Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur J Cancer 2017;82:56-65. [Crossref] [PubMed]
- Bender C, Enk A, Gutzmer R, et al. Anti-PD-1 antibodies in metastatic uveal melanoma: a treatment option? Cancer Med 2017;6:1581-6. [Crossref] [PubMed]
- Algazi AP, Tsai KK, Shoushtari AN, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016;122:3344-53. [Crossref] [PubMed]
- Kottschade LA, McWilliams RR, Markovic SN, et al. The use of pembrolizumab for the treatment of metastatic uveal melanoma. Melanoma Res 2016;26:300-3. [Crossref] [PubMed]
- Piperno-Neumann S, Servois V, Mariani P, et al. Activity of anti-PD1 drugs in uveal melanoma patients. J Clin Oncol 2016;34:9588.
- Karydis I, Chan PY, Wheater M, et al. Clinical activity and safety of pembrolizumab in ipilimumab pre-treated patients with uveal melanoma. Oncoimmunology 2016;5:e1143997. [Crossref] [PubMed]
- Schadendorf D, Ascierto PA, Haanen J, et al. Efficacy and safety of nivolumab (NIVO) in patients with advanced melanoma (MEL) and poor prognostic factors who progressed on or after ipilimumab (IPI): results from a phase II study (CheckMate 172). J Clin Oncol 2017.35.
- Shoushtari AN, Navld-Azarbaljanl P, Friedman CF, et al. Efficacy of nivolumab and ipilimumab (Nivo + Ipi) combination in melanoma patients (pts) treated at a single institution on an expanded-access program (EAP). J Clin Oncol 2016;34:9554.
- van der Kooij MK, Joosse A, Speetjens FM, et al. Anti-PD1 treatment in metastatic uveal melanoma in the Netherlands. Acta Oncol 2017;56:101-3. [Crossref] [PubMed]
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109-17. [Crossref] [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. [Crossref] [PubMed]
- Bol KF, Mensink HW, Aarntzen EH, et al. Long overall survival after dendritic cell vaccination in metastatic uveal melanoma patients. Am J Ophthalmol 2014;158:939-47. [Crossref] [PubMed]
- Kawakami Y, Eliyahu S, Delgado CH, et al. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci U S A 1994;91:6458-62. [Crossref] [PubMed]
- Coulie PG, Brichard V, Van Pel A, et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med 1994;180:35-42. [Crossref] [PubMed]
- Kawakami Y, Eliyahu S, Delgado CH, et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci U S A 1994;91:3515-9. [Crossref] [PubMed]
- Brichard V, Van Pel A, Wölfel T, et al. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med 1993;178:489-95. [Crossref] [PubMed]
- Robbins PF, El Gamil M, Kawakami Y, et al. Recognition of tyrosinase by tumor-infiltrating lymphocytes from a patient responding to immunotherapy. Cancer Res 1994;54:3124-6. [PubMed]
- van Dinten LC, Pul N, van Nieuwpoort AF, et al. Uveal and cutaneous melanoma: shared expression characteristics of melanoma-associated antigens. Invest Ophthalmol Vis Sci 2005;46:24-30. [Crossref] [PubMed]
- Middleton MR, Steven NM, Evans TJ, et al. Safety, pharmacokinetics and efficacy of IMCgp100, a first-in-class soluble TCR-antiCD3 bispecific t cell redirector with solid tumour activity: Results from the FIH study in melanoma. J Clin Oncol 2016;34:3016.
- Shoushtari AN, Evans J, Corrie P, et al. editors. A phase I study of IMCgp100, a soluble HLA-A2 restricted gp100-specific Tcell receptor-CD3 therapeutic with solid tumor activity in patients with advanced uveal melanoma. Late-breaking Abstract and Oral Presentation at the Society for Melanoma Research Congress. 6-9 November, 2016; Boston, Massachusetts.
- Field MG, Durante MA, Decatur CL, et al. Epigenetic reprogramming and aberrant expression of PRAME are associated with increased metastatic risk in Class 1 and Class 2 uveal melanomas. Oncotarget 2016;7:59209-19. [Crossref] [PubMed]
- Field MG, Decatur CL, Kurtenbach S, et al. PRAME as an Independent Biomarker for Metastasis in Uveal Melanoma. Clin Cancer Res 2016;22:1234-42. [Crossref] [PubMed]
- Chang A, Dao T, Scott A, et al. A therapeutic TCR mimic monoclonal antibody for intracellular PRAME protein in leukemias. Blood 2015;126:2527.
- Amir AL, van der Steen DM, van Loenen MM, et al. PRAME-specific Allo-HLA-restricted T cells with potent antitumor reactivity useful for therapeutic T-cell receptor gene transfer. Clin Cancer Res 2011;17:5615-25. [Crossref] [PubMed]