Pathologic classification of “pancreatic cancers”: current concepts and challenges
Introduction
Since pancreatic ductal adenocarcinoma (PDAC) accounts for the vast majority of the cancers of the pancreas, the term “pancreas cancer” is commonly used interchangeably to refer to PDAC (1-3). However, in reality there are diverse types of primary as well as secondary malignant neoplasms that can be encountered in the pancreas with various clinicopathologic characteristics and biologic behavior, including more benevolent entities like solid pseudopapillary neoplasms (SPNs), or cancers with more indolent behavior like neuroendocrine tumors (NET) as well as relatively aggressive cancers like acinar type, which also show substantial differences from PDAC (Table 1). Even ordinary PDACs are now proving to have previously unrecognized subsets in molecular profiling studies (2,4-7). In the ensuing text, the salient features of these entities and new developments will be discussed.
Full table
PDAC: the pancreatic cancer
PDAC is by far the most common type of primary malignant neoplasm of the pancreas and thus most attributes of “pancreas cancer” are related to this tumor type. That is why PDAC is also referred to as “ordinary” or “conventional” pancreatic adenocarcinoma.
Histologically (microscopically), PDAC is by definition a “pancreatobiliary type” adenocarcinoma, and in fact, is the defining tumor type for this category which also encompasses gallbladder and cholangiocarcinoma.
It is this tumor type that accounts for more than 85% of the primary pancreatic cancers and is very uncommon in patients under 30 years old (mean age of presentation 63). It is the 3rd leading cause of cancer deaths in USA, with 5-year overall survival rate of 5–10% (8-10).
PDACs are significantly more common in the head than the rest of the pancreas (11). The vast majority are solid tumors although occasionally they present as a cystic mass or show some cystic areas (12,13). PDAC tends to infiltrate the surrounding tissue very insidiously without forming a compact mass lesion, with size at presentation less than 7.0 cm in the vast majority of cases (14). Also by the time it reaches to significant size at the primary site, PDAC is already widely disseminated. In fact, a compact well-formed mass lesion in the pancreas that is larger than a few centimeters is very unlikely to be a PDAC unless it is in the tail portion of the organ (14,15).
PDACs commonly involve the duodenum, or ampulla, which poses challenges in determining the precise origin of the tumor (16,17). In fact, this is currently one of the most problematic aspects in pancreatic cancer research especially in an era where low-frequency “subtypes” of “PDAC” are identified for targeted therapies; however, many of the cases identified prove to be secondary from the ampulla or duodenum. Proper dissection of the pancreatoduodenectomy specimens, with careful correlation with gross and clinical findings is crucial in establishing the primary site by determining the epicenter of the tumor. PDACs commonly involve the common bile duct (CBD) (in more than 90% of the cases) (15), and that is why, for a tumor involving the CBD, a purist’s approach should be employed before a case can be classified as intrapancreatic CBD carcinoma (18).
Microscopically, being pancreatobiliary type adenocarcinomas, classical PDACs are characterized by tubules/ducts (Figure 1), which are sometimes cytologically bland to a degree making their distinction from reactive ducts of chronic pancreatitis highly challenging, especially given the fact that chronic injury also commonly elicits substantial epithelial atypia that can be impossible to distinguish from carcinoma. In fact, this is regarded as one of the most challenging differential diagnosis in diagnostic pathology.
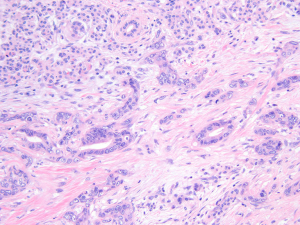
It is well known that PDAC has a very high propensity to exhibit perineural invasion. Additionally, it is also becoming clear that PDAC also shows very high predilection to invade vascular channels and form duct-like structures by lining the endothelium (19,20). This phenomenon is now being appreciated as a very common occurrence, but is underestimated in histologic examinations and markedly underreported. This also appears to be a mode of spread that may be contributing to the local dissemination and very aggressive behavior of PDACs.
Despite the earlier studies claiming that PDAC may take years to develop, it is becoming clear that the progression of PDAC, once it forms, seems to be a wild-fire phenomenon, in the sense that, once it starts, it very rapidly spreads. For this reason, PDACs are seldom detected when they are very small even in screening protocols (10).
For staging of pancreas cancers, AJCC/UICC (8th edition) for 2018 adopted several modifications. For T-staging, the new protocol eliminated the irreproducible and ill-defined parameters such as “peripancreatic soft tissue invasion” (15) and replaced it solely by tumor size. Additionally, number of lymph nodes with metastasis also has been found to be a prognosticator (21) and thus included in the new staging as N1 and N2.
At the immunohistochemical level, PDACs show foregut phenotype with CK7 positivity. They are by definition mucin producing cells, and mucin-related glycoproteins/oncoproteins such as CEA, CA19-9, MUC1, MUC4, MUC5AC are expressed in abundance. However, these are also expressed in other upper GI cancers as well as lung cancers, and, to some degree even in GYN-tract gynecologic adenocarcinomas. Loss of SMAD4 is seen in about a half to two-third of the cases, however, is quite uncommon in other cancer types and may be helpful in the differential diagnosis (22,23). Also, p53 and S100P protein can play a role in the differentiation of PDACs from its benign mimickers like chronic pancreatitis, if used cautiously (24).
It is generally stated that “cancer is a genetic disease”. Mutation in the codon 12 of kras oncogene is identified in the vast majority of PDACs in carefully conducted studies (22), and kras mutation is believed to create the baseline abnormality in the ductal epithelium upon which other mutations such as P53, DPC4 (SMAD4), p16 and others ensue, which in turn further drive the carcinogenesis into full-blown cancer. Several germline genetic alterations have been identified to be causally related to PDAC, such as P16INK4A/CDKN2A (familial atypical multiple mole melanoma syndrome), STK11/LKB1 (Peutz-Jeghers syndrome), and BRCA mutations (10). Recent molecular and proteomic expression profiling studies have also identified distinct clusters of PDAC previously unrecognized (2,4-6). For example, in the study by Bailey et al. (6), a “basal-like” category similar to that in the breast was delineated, which appears to be related to adenosquamous carcinomas (see below). The other groups require further verification. It should be reiterated here however that some of the subsets identified appear to be non-ductal cancers, cancers of neighboring sites and even abnormalities of chronic pancreatitis. Further studies are needed to clarify these issues.
PDAC’s histomorphologic variants
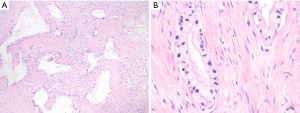
While most PDACs are characterized by small tubular units with a distinctive cytology, there are variations in this theme that worth notation because they sometimes lead to misdiagnosis in histologic examination. They are presumably products of some variation in the molecular-genetic pathways, but the significance of these variants has yet to be determined (25). In some PDACs, the infiltrating tubules are fairly large and closely mimic intraductal or cystic neoplasia (26,27). Such tumors seem to have slightly better behavior. Similarly, some PDACs are deceptively bland-appearing with mature glandular structures showing abundant foamy/microvesicular cytoplasm (Figure 2) (foamy gland variant) (28). Yet other PDACs exhibit prominent intracytoplasmic vacuole formation (29); it is not known whether this pattern is associated with specific secretory activity and higher CA19-9 levels. This pattern is unusual in carcinomas of other organs and thus can be helpful in the diagnosis of PDAC at metastatic sites.
PDAC related carcinomas that are classified separately
There are carcinoma types that are closely related to PDACs and are often accompanied by ordinary PDAC foci within the same tumor, but are regarded as separate entities because of their biologic connotations.
One of these is adenosquamous carcinoma, which rare type accounts for 1–4% of exocrine pancreatic malignancies often present as necrotic masses (30). In biopsies, especially in fine needle aspiration (FNA) specimens, the unexpected squamous changes can lead to the impression of metastasis or other tumor types. Recent molecular profiling studies have identified adenosquamous carcinomas as a separate (“basal type”) cluster from ordinary ductal adenocarcinomas which is not surprising (6). In cytologic and histologic preparations, adenosquamous differentiation can be highlighted by differential expression of basal type keratins (such as CK5/6) as well as P63 and/or P40.
Another category is osteoclastic giant cell carcinoma, which is also often but not always accompanied by ordinary PDAC. This type is rare and accounts for 1.4% of invasive pancreatic cancer (31). This tumor also appears to have a different molecular profile, captured as a separate cluster in the profiling studies. It appears to be a distinct type of sarcomatoid carcinoma (ultimate example of epithelial-mesenchymal transition) in which the tumor cells somehow attract osteoclasts (bone resorption cells) in abundance. They are often demarcated tumors that commonly arise in association with mucinous cystic neoplasms or intraductal papillary mucinous neoplasms (IPMNs) (in fact, they often present as polypoid masses in the ducts or cysts). Their prognosis seems to be significantly better than that of ordinary PDACs although in one study, the cases that had undergone prior FNA/biopsy were found to have aggressive behavior (32).
Other carcinoma types of ductal origin but classified separately from PDAC
There are other carcinoma types that arise from the ductal system (or exhibit ductal differentiation as PDACs), but have distinct morphologic, immunophenotypic, molecular, and most importantly, biologic behavior than PDACs and thus ought to be classified separately (14).
Colloid (pure mucinous) carcinoma is a rare tumor (approximately 1–3% of malignant neoplasms of the exocrine pancreas) (33), of ductal origin, and as such technically a relative of the PDAC, but in fact is a carcinoma with a markedly protracted clinical course incomparably better than that of PDAC, with a 5-year survival >55% (34,35). This is also true for colloid carcinomas of other exocrine organs (breast and skin) where colloid carcinoma has a very indolent behavior. In the pancreas too, this tumor can have long protracted clinical course even when it is large in size and/or already metastasized to lymph nodes. The speculation has been that the mucin produced engulfs the tumor cells and serve as a containing factor, preventing the tumor cells from spreading. As importantly, colloid carcinoma of the pancreas is a tumor with clear-cut intestinal differentiation, showing diffuse expression of intestinal programming transcription factor CDX2, and goblet cell marker MUC2, both of which are not detectable in any pancreatic tumor (certainly not in PDACs) other than intestinal variant of IPMNs from which colloid carcinomas are often seen arising from (36). For these reasons, it may be good to view a colloid carcinoma as a type of “intestinal” carcinoma rather than a conventional “pancreatobiliary” carcinoma and treat it as such.
Another carcinoma that can occasionally arise in the pancreas and is presumed to be of ductal origin is medullary carcinoma (37). This tumor is defined as it is in the ampulla/duodenum or lower GI tract, characterized by nodular (demarcated) syncytial growth pattern, pushing border infiltration and inflammatory infiltrates, and common association with microsatellite instability. Defined as such, medullary carcinoma is exceedingly uncommon in the pancreas proper, and most cases classified as medullary carcinoma in this region are actually of ampullary/duodenal origin. These tumors appear to be very much akin to their GI tract counterparts and it is assumed that they warrant management as a medullary carcinoma of the GI tract. They are commonly associated with microsatellite instability (37-40).
Carcinomas arising in tumoral intraepithelial neoplasms
Most PDACs arise “de-novo”, presumably (and commonly) in association with microscopic/incidental forms of dysplasia that are termed PanIN in the pancreas. However, an estimated 4% of PDACs (with the conventional PDAC morphology and behavior) arise in a tumoral intraepithelial neoplasm, namely IPMNs or mucinous cystic neoplasms (41).
The exact frequency of invasive carcinoma in IPMNs is difficult to gauge because of the selection bias in studies that has also been changing dramatically over time. It is generally estimated that 10–15% of IPMNs will prove to harbor invasive carcinoma in resection, or develop it in the follow up (42-44). Until recently, this figure was “30%” in studies focusing on resection databases.
The invasive carcinomas arising from IPMNs can be either of ordinary PDAC or colloid carcinoma types, the latter with a different biology and much more protracted clinical course as discussed above (34,35). Colloid carcinoma is typically associated with intestinal type IPMNs, with both tumors abundantly expressing intestinal lineage markers (MUC2 and CDX2) which are otherwise seldom seen in other pancreatic carcinomas. Additionally, mutation in GNAS that has been found in IPMNs also appears to be most closely related to the intestinal subtype (45-47).
Gastric type IPMNs are the most common variant of IPMNs, with most “incidentaloma” cysts (which are detected in about 10% of the elderly population) proving in fact of this type. While invasion is relatively uncommon in this type of IPMN, when it occurs, it is typically of the tubular (PDAC) type (48-50). The tubular PDACs arising from IPMNs seem to be not only identical morphologically to de-novo PDACs but also show aggressive behavior, although, recent studies have shown that these IPMN-associated tubular adenocarcinomas seem to behave slightly better than de-novo PDACs (51-53) even when stage matched.
Oncocytic variant of IPMNs (54-58), which are also called IOPNs (intraductal oncocytic papillary neoplasms) often present as highly complex partially cystic and partially solid masses, and they also often give the impression of an unresectable tumor. Moreover, they often are misdiagnosed as “adenocarcinoma” on cytological preparations based on the striking atypia (55). Thus, they often come to the attention of oncologists. However, despite their complexity and seemingly infiltrative appearance, they are in fact surgically curable tumors with estimated 10-year survival about 90%. The molecular profile of these tumors is different than those of IPMNs (56). They lack KRAS and GNAS mutations that are otherwise fairly common in other IPMNs. They also express heppar1, mesothelin and MUC6 significantly more commonly than others (59). Not surprisingly, they typically remain stable (do not respond to) cytotoxic chemotherapy protocols since they are low proliferative and slow growing.
Intraductal tubular (tubulo-papillary) neoplasm (ITPN) is increasingly being recognized as a distinct category both in the pancreas (60,61) and biliary tract (62). These tumors are similar to IOPNs in their complexity and proneness to be diagnosed as an aggressive “adenocarcinoma” although they seem to be fairly indolent tumors with a protracted clinical course. They are distinguished from IPMNs and IOPNs by their tubular configuration, lack of mucin production, as well as their molecular and immunophenotypic background (61). These tumors are also genetically and molecularly distinct from IPMNs. A percentage of cases show loss of P16INK4A/CDKN2A and mutations in chromatin modelling genes, but perhaps more importantly, phosphatidylinositol 3-kinase (PI3K) pathway is often altered, thus making some of these patients candidate for targeted therapy (61).
Mucinous cystic neoplasms are another type of tumor with adenoma-carcinoma sequence (tumoral intraepithelial neoplasms), with about 16% of the resected mucinous cystic neoplasms (MCNs) (63) showing invasive adenocarcinoma. Once these tumors are defined by the ovarian type stroma, 98% are seen in peri-menopausal women and >90% arise in the tail of the organ. The vast majority of invasive carcinomas that arise in MCNs are of the tubular/ductal type (i.e., PDACs), and their behavior is also very similar to PDACs. The cases with very small (microscopic, pT1a/b) carcinomas appear to be curable; however, we have seen cases that progressed aggressively even when the invasion was small. Invasion into peri-cystic tissues appear to be a factor in more adverse outcome. MCNs can also lead to osteoclastic giant cell carcinomas discussed above.
Non-ductal cancers
While the vast majority of malignant pancreatic neoplasms are those with ductal lineage (discussed above), about 5% are non-ductal tumors, which will be reviewed briefly below.
Pancreatic neuroendocrine neoplasms (PanNENs)
PanNENs are now regarded in two distinct categories per WHO 2017. One is the well-differentiated NETs (WDNET), what used to be known as “islet cell tumors/carcinomas”, and the other are the poorly differentiated neuroendocrine carcinomas (PDNEC) as defined in the lung, with small cell and large cell variants (64,65). These two groups appear to be strikingly different both by biology, clinical behavior and molecular phenotype.
Well differentiated NETs are low grade malignancies which are curable if removed when they are low-grade/low-stage. Even when they are metastatic, they are often slow-growing. But with the same token, they do not respond to therapy well (66-68). Microscopically, these tumors have easily recognizable neuroendocrine morphology that is similar to carcinoids of GI tract or the lung, composed of nests and trabeculae of monotonous cells with fair amount of cytoplasm and round nuclei with the distinctive “salt-and-pepper” chromatin. Typically, Ki67 proliferation is low, with the vast majority, <10%. WDNETs are graded as G1 or G2 based on Ki67 index of less or more than 3.0. Occasionally, a pancreatic WDNET with the classical well-differentiated morphology can have Ki67 >20%, and these cases which were designated “grade discordant” category previously (69) are now classified as G3, and their prognosis is more aggressive than lesser PanNENs, but still significantly better than PDNECs.
Poorly differentiated NE carcinomas are defined by their high-grade morphology, which is very similar to their pulmonary counterparts, and they are very aggressive clinically. Their Ki67 index is typically way above 50% range. These tumors show striking initial sensitivity to cis-platinum-based treatments but unfortunately recur soon after, and display an overall dismal prognosis.
Increasingly, gray zone cases between a WDNET and PDNEC are being recognized and present as a classification challenge. These cases have been termed “ambiguous” (70) for which immunohistophenotype may reveal the true identity. P53 overexpression and loss of RB are significantly more common in PDNEC, and exceedingly uncommon in WDNETs. In contrast, loss of ATRX/DAXX, which is seen in close to half of WDNETs (70,71) is not a feature of PDNECs. Additionally, Ki67 proliferation index is also much higher in the true PDNECs.
Acinar cell carcinomas
Acinar cell carcinomas are relatively rare. They are characterized by recapitulation of acinar cells (Figure 3), showing enzymatic granules both histologically (manifested as granules or cytoplasmic chromophilia), and monotonous nuclei with single prominent nucleoli, and immunohistochemically, they show positivity for acinar enzymes trypsin, chymotrypsin and lipases (72-74). Mutations that are frequent in PDACs such as KRAS, p16, and SMAD4 mutations are typically low in acinar cell carcinomas (74,75). P53 appears to be altered in some but through a mechanism different than in PDAC.
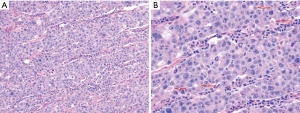
Acinar carcinomas can be regarded as “intermediate grade” cancers in the spectrum of pancreatic cancers. Their Ki67 index is typically between 10–50% range, but is much higher in some cases. They have an aggressive behavior, however, their overall 5-year survival seem to be much better than ordinary PDACs (76,77). They are often large, compact and demarcated tumors, commonly associated with necrosis.
Pancreatoblastomas
Pancreatoblastoma is a distinctive tumor type that occurs in the pancreas the hallmark of which is the presence of the so-called squamoid corpuscles (morules) that show nuclear beta-catenin accumulation (78-80). Typically, true to their name “blastoma”, they show a mixture of cell lineages, predominantly acinar but also neuroendocrine and even ductal. They are often misdiagnosed as NETs or acinar tumors. Pancreatoblastomas can be associated with familial adenomatous polyposis (FAP) and related syndromes (including Gardner), and the expression of beta-catenin in the morules is also a side manifestation of the cellular alterations in the Wnt (APC/beta-catenin) pathway (80-82). Although they are generally thought to be tumors of childhood, in fact, a substantial proportion of pancreatoblastomas are seen in middle-aged adults. Limited data indicates that they are close kindreds of acinar cell carcinomas (ACCs) both by morphology, molecular profile, as well as behavior.
Solid pseudo-papillary neoplasm (SPN)
SPN is a very peculiar tumor specific to pancreas (very rare examples have been reported at extra-pancreatic sites). The cell of origin or cell lineage of this tumor is unclear. Although it had been designated as “carcinoma” in the past, even its epithelial nature is dubious with most examples showing minimal if any keratin expression (83).
SPNs can be very difficult to distinguish from NETs both at clinical and pathologic level. In fact, they often get misdiagnosed. However, immunohistochemical stains are very helpful in this distinction, with beta-catenin typically showing diffuse nuclear labeling in SPNs, while keratins and chromogranin both of which are commonly either negative or focal positive in SPNs (84,85). SPNs also typically express progesterone receptors (as do some NETs), which, combined with the fact that most (though not all) patients are young females, raise the possibility of hormone related initiation and progression for these tumors, and a potential target for inhibitors of related pathways. Although SPNs are regarded under “malignant” category, and therefore staged accordingly, they in fact very seldom exhibit malignant behavior even when they are large and advanced. In fact, even the cases with liver metastasis can be curable. Along those lines, many cases that are classified as metastatic SPNs or rapidly progressive SPNs prove to be some other tumor type under careful scrutiny of the pathology. In carefully performed studies, the 10-year survival of SPNs is >95%, and the few mortalities reported carry questions about the accuracy of the diagnosis (83,86).
Secondary tumors
One of the most challenging aspects of pathologic classification and proper management of pancreatic cancers is the problem in distinguishing them from secondary malignancies, both clinically and pathologically. Ampullary/duodenal and CBD cancers often secondarily invade the pancreas and present like a primary of the pancreas. In fact, an unusual cancer type such as signet ring, mucinous or medullary occurring in this region should be regarded as ampullary or duodenal rather than pancreatic unless proven otherwise. Proper dissection of the pancreatoduodenectomy specimens with careful attention to the recently refined criteria (16,87) is crucial. The grossing protocol recommended by Verbeke et al. (88) and increasingly being adopted in Europe appears to be problematic in this regard.
There are a variety of secondary tumors metastatic to pancreas that can mimic primary cancers, too (89). Among these especially lymphomas, renal cell carcinomas (RCCs) and gastric cancers are prone to be misdiagnosed as primary pancreatic adenocarcinoma pre-operatively. For metastatic RCCs, the pancreatic tumor may appear decades after the original diagnosis and thus not considered in the differential diagnosis. It also appears that the resection of a metastatic RCC in the pancreas can achieve long term survival in some patients (90,91).
Mesenchymal neoplasms
Virtually every sarcoma type can occur in the pancreas. The most common ones appear to be GISTs (many of which are secondary involvement from duodenum and stomach, although some may be true primary), and leiomyosarcomas (92-94).
Pseudotumors
Studies have shown that a variety of benign conditions can mimic pancreas cancer and lead to misdiagnosis and unnecessary management (95). Among these, especially paraduodenal (groove) pancreatitis (96) and autoimmune pancreatitis (97) are notorious for being mistaken as a primary cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Klimstra DS, Adsay NV. Benign and malignant tumors of the pancreas. In: Odze R, Goldblum JR, Crawford JM, editors. Surgical pathology of the GI tract, liver, biliary tract and pancreas. Philadelphia, PA: Saunders, 2004.
- Chiaravalli M, Reni M, O’Reilly EM. Pancreatic ductal adenocarcinoma: State-of-the-art 2017 and new therapeutic strategies. Cancer Treat Rev 2017;60:32-43. [Crossref] [PubMed]
- Jemal A, Ward EM, Johnson CJ, et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst 2017.109. [PubMed]
- Donahue TR, Tran LM, Hill R, et al. Integrative survival-based molecular profiling of human pancreatic cancer. Clin Cancer Res 2012;18:1352-63. [Crossref] [PubMed]
- Humphrey ES, Su SP, Nagrial AM, et al. Resolution of Novel Pancreatic Ductal Adenocarcinoma Subtypes by Global Phosphotyrosine Profiling. Mol Cell Proteomics 2016;15:2671-85. [Crossref] [PubMed]
- Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47-52. [Crossref] [PubMed]
- Schlitter AM, Segler A, Steiger K, et al. Molecular, morphological and survival analysis of 177 resected pancreatic ductal adenocarcinomas (PDACs): Identification of prognostic subtypes. Sci Rep 2017;7:41064. [Crossref] [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. Contents of the SEER Cancer Statistics Review (CSR), 1975-2014. Bethesda: National Cancer Institute, 2017. Available online: https://seer.cancer.gov/csr/1975_2014/sections.html
- Sirri E, Castro FA, Kieschke J, et al. Recent Trends in Survival of Patients With Pancreatic Cancer in Germany and the United States. Pancreas 2016;45:908-14. [Crossref] [PubMed]
- Becker AE, Hernandez YG, Frucht H, et al. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol 2014;20:11182-98. [Crossref] [PubMed]
- Artinyan A, Soriano PA, Prendergast C, et al. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford) 2008;10:371-6. [Crossref] [PubMed]
- Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med 2009;133:423-38. [PubMed]
- Adsay NV. Cystic neoplasia of the pancreas: pathology and biology. J Gastrointest Surg 2008;12:401-4. [Crossref] [PubMed]
- Thompson LD, Basturk O, Adsay V. Pancreas. In: Mills SE, Greenson JK, Hornick JL, et al., editors. Sternberg’s diagnostic surgical pathology: sixth edition. Wolters Kluwer Health Adis (ESP), 2015.
- Saka B, Balci S, Basturk O, et al. Pancreatic Ductal Adenocarcinoma is Spread to the Peripancreatic Soft Tissue in the Majority of Resected Cases, Rendering the AJCC T-Stage Protocol (7th Edition) Inapplicable and Insignificant: A Size-Based Staging System (pT1: ≤2, pT2: >2-≤4, pT3: >4 cm) is More Valid and Clinically Relevant. Ann Surg Oncol 2016;23:2010-8.
- Adsay V, Ohike N, Tajiri T, et al. Ampullary region carcinomas: definition and site specific classification with delineation of four clinicopathologically and prognostically distinct subsets in an analysis of 249 cases. Am J Surg Pathol 2012;36:1592-608. [Crossref] [PubMed]
- Xue Y, Vanoli A, Balci S, et al. Non-ampullary-duodenal carcinomas: clinicopathologic analysis of 47 cases and comparison with ampullary and pancreatic adenocarcinomas. Mod Pathol 2017;30:255-66. [Crossref] [PubMed]
- Gonzalez RS, Bagci P, Basturk O, et al. Intrapancreatic distal common bile duct carcinoma: Analysis, staging considerations, and comparison with pancreatic ductal and ampullary adenocarcinomas. Mod Pathol 2016;29:1358-69. [Crossref] [PubMed]
- Bandyopadhyay S, Basturk O, Coban I, et al. Isolated solitary ducts (naked ducts) in adipose tissue: a specific but underappreciated finding of pancreatic adenocarcinoma and one of the potential reasons of understaging and high recurrence rate. Am J Surg Pathol 2009;33:425-9. [Crossref] [PubMed]
- Hong SM, Goggins M, Wolfgang CL, et al. Vascular invasion in infiltrating ductal adenocarcinoma of the pancreas can mimic pancreatic intraepithelial neoplasia: a histopathologic study of 209 cases. Am J Surg Pathol 2012;36:235-41. [Crossref] [PubMed]
- Basturk O, Saka B, Balci S, et al. Substaging of Lymph Node Status in Resected Pancreatic Ductal Adenocarcinoma Has Strong Prognostic Correlations: Proposal for a Revised N Classification for TNM Staging. Ann Surg Oncol 2015;22 Suppl 3:S1187-95. [Crossref] [PubMed]
- Hruban RH, Iacobuzio-Donahue C, Wilentz RE, et al. Molecular pathology of pancreatic cancer. Cancer J 2001;7:251-8. [PubMed]
- Schutte M. DPC4/SMAD4 gene alterations in human cancer, and their functional implications. Ann Oncol 1999;10 Suppl 4:56-9. [Crossref] [PubMed]
- Liu H, Shi J, Anandan V, et al. Reevaluation and identification of the best immunohistochemical panel (pVHL, Maspin, S100P, IMP-3) for ductal adenocarcinoma of the pancreas. Arch Pathol Lab Med 2012;136:601-9. [Crossref] [PubMed]
- Verbeke C. Morphological heterogeneity in ductal adenocarcinoma of the pancreas - Does it matter? Pancreatology 2016;16:295-301. [Crossref] [PubMed]
- Bagci P, Andea AA, Basturk O, et al. Large duct type invasive adenocarcinoma of the pancreas with microcystic and papillary patterns: a potential microscopic mimic of non-invasive ductal neoplasia. Mod Pathol 2012;25:439-48. [Crossref] [PubMed]
- Kelly PJ, Shinagare S, Sainani N, et al. Cystic papillary pattern in pancreatic ductal adenocarcinoma: a heretofore undescribed morphologic pattern that mimics intraductal papillary mucinous carcinoma. Am J Surg Pathol 2012;36:696-701. [Crossref] [PubMed]
- Adsay V, Logani S, Sarkar F, et al. Foamy gland pattern of pancreatic ductal adenocarcinoma: a deceptively benign-appearing variant. Am J Surg Pathol 2000;24:493-504. [Crossref] [PubMed]
- Dursun N, Feng J, Basturk O, et al. Vacuolated cell pattern of pancreatobiliary adenocarcinoma: a clinicopathological analysis of 24 cases of a poorly recognized distinctive morphologic variant important in the differential diagnosis. Virchows Arch 2010;457:643-9. [Crossref] [PubMed]
- Madura JA, Jarman BT, Doherty MG, et al. Adenosquamous carcinoma of the pancreas. Arch Surg 1999;134:599-603. [Crossref] [PubMed]
- Muraki T, Reid MD, Basturk O, et al. Undifferentiated Carcinoma With Osteoclastic Giant Cells of the Pancreas: Clinicopathologic Analysis of 38 Cases Highlights a More Protracted Clinical Course Than Currently Appreciated. Am J Surg Pathol 2016;40:1203-16. [Crossref] [PubMed]
- Reid MD, Muraki T. Cytologic features and clinical implications of undifferentiated carcinoma with osteoclastic giant cells of the pancreas: An analysis of 15 cases. Cancer 2017;125:563-75. [PubMed]
- Liszka L, Zielinska-Pajak E, Pajak J, et al. Colloid carcinoma of the pancreas: review of selected pathological and clinical aspects. Pathology 2008;40:655-63. [Crossref] [PubMed]
- Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol 2001;25:26-42. [Crossref] [PubMed]
- Adsay NV, Merati K, Nassar H, et al. Pathogenesis of colloid (pure mucinous) carcinoma of exocrine organs: Coupling of gel-forming mucin (MUC2) production with altered cell polarity and abnormal cell-stroma interaction may be the key factor in the morphogenesis and indolent behavior of colloid carcinoma in the breast and pancreas. Am J Surg Pathol 2003;27:571-8. [Crossref] [PubMed]
- Adsay NV, Merati K, Andea A, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol 2002;15:1087-95. [Crossref] [PubMed]
- Wilentz RE, Goggins M, Redston M, et al. Genetic, immunohistochemical, and clinical features of medullary carcinoma of the pancreas: A newly described and characterized entity. Am J Pathol 2000;156:1641-51. [Crossref] [PubMed]
- Banville N, Geraghty R, Fox E, et al. Medullary carcinoma of the pancreas in a man with hereditary nonpolyposis colorectal cancer due to a mutation of the MSH2 mismatch repair gene. Hum Pathol 2006;37:1498-502. [Crossref] [PubMed]
- Kondo E, Furukawa T, Yoshinaga K, et al. Not hMSH2 but hMLH1 is frequently silenced by hypermethylation in endometrial cancer but rarely silenced in pancreatic cancer with microsatellite instability. Int J Oncol 2000;17:535-41. [PubMed]
- Yamamoto H, Itoh F, Nakamura H, et al. Genetic and clinical features of human pancreatic ductal adenocarcinomas with widespread microsatellite instability. Cancer Res 2001;61:3139-44. [PubMed]
- Adsay V, Mino-Kenudson M, Furukawa T, et al. Pathologic Evaluation and Reporting of Intraductal Papillary Mucinous Neoplasms of the Pancreas and Other Tumoral Intraepithelial Neoplasms of Pancreatobiliary Tract: Recommendations of Verona Consensus Meeting. Ann Surg 2016;263:162-77. [Crossref] [PubMed]
- Terris B, Ponsot P, Paye F, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol 2000;24:1372-7. [Crossref] [PubMed]
- Salvia R, Fernández-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg 2004;239:678-85; discussion 685-7. [Crossref] [PubMed]
- Sugiyama M, Izumisato Y, Abe N, et al. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg 2003;90:1244-9. [Crossref] [PubMed]
- Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011;3:92ra66. [Crossref] [PubMed]
- Furukawa T, Kuboki Y, Tanji E, et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep 2011;1:161. [Crossref] [PubMed]
- Tan MC, Basturk O, Brannon AR, et al. GNAS and KRAS Mutations Define Separate Progression Pathways in Intraductal Papillary Mucinous Neoplasm-Associated Carcinoma. J Am Coll Surg 2015;220:845-54.e1. [Crossref] [PubMed]
- Lüttges J, Zamboni G, Longnecker D, et al. The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol 2001;25:942-8. [Crossref] [PubMed]
- Adsay NV, Conlon KC, Zee SY, et al. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer 2002;94:62-77. [Crossref] [PubMed]
- Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an "intestinal" pathway of carcinogenesis in the pancreas. Am J Surg Pathol 2004;28:839-48. [Crossref] [PubMed]
- Shimada K, Sakamoto Y, Sano T, et al. Invasive carcinoma originating in an intraductal papillary mucinous neoplasm of the pancreas: a clinicopathologic comparison with a common type of invasive ductal carcinoma. Pancreas 2006;32:281-7. [Crossref] [PubMed]
- Yopp AC, Katabi N, Janakos M, et al. Invasive carcinoma arising in intraductal papillary mucinous neoplasms of the pancreas: a matched control study with conventional pancreatic ductal adenocarcinoma. Ann Surg 2011;253:968-74. [Crossref] [PubMed]
- Murakami Y, Uemura K, Sudo T, et al. Invasive intraductal papillary-mucinous neoplasm of the pancreas: comparison with pancreatic ductal adenocarcinoma. J Surg Oncol 2009;100:13-8. [Crossref] [PubMed]
- Adsay NV, Longnecker DS, Klimstra DS. Pancreatic tumors with cystic dilatation of the ducts: intraductal papillary mucinous neoplasms and intraductal oncocytic papillary neoplasms. Semin Diagn Pathol 2000;17:16-30. [PubMed]
- Reid MD, Stallworth CR, Lewis MM, et al. Cytopathologic diagnosis of oncocytic type intraductal papillary mucinous neoplasm: Criteria and clinical implications of accurate diagnosis. Cancer Cytopathol 2016;124:122-34. [Crossref] [PubMed]
- Basturk O, Tan M, Bhanot U, et al. The oncocytic subtype is genetically distinct from other pancreatic intraductal papillary mucinous neoplasm subtypes. Mod Pathol 2016;29:1058-69. [Crossref] [PubMed]
- Basturk O, Khayyata S, Klimstra DS, et al. Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pyloropancreatic pathway, distinct from the intestinal pathway, in pancreatic carcinogenesis. Am J Surg Pathol 2010;34:364-70. [Crossref] [PubMed]
- Marchegiani G, Mino-Kenudson M, Ferrone CR, et al. Oncocytic-type intraductal papillary mucinous neoplasms: a unique malignant pancreatic tumor with good long-term prognosis. J Am Coll Surg 2015;220:839-44. [Crossref] [PubMed]
- Basturk O, Chung SM, Hruban RH, et al. Distinct pathways of pathogenesis of intraductal oncocytic papillary neoplasms and intraductal papillary mucinous neoplasms of the pancreas. Virchows Arch 2016;469:523-32. [Crossref] [PubMed]
- Basturk O, Adsay V, Askan G, et al. Intraductal Tubulopapillary Neoplasm of the Pancreas: A Clinicopathologic and Immunohistochemical Analysis of 33 Cases. Am J Surg Pathol 2017;41:313-25. [Crossref] [PubMed]
- Basturk O, Berger MF, Yamaguchi H, et al. Pancreatic intraductal tubulopapillary neoplasm is genetically distinct from intraductal papillary mucinous neoplasm and ductal adenocarcinoma. Mod Pathol 2017;30:1760-72. [Crossref] [PubMed]
- Schlitter AM, Jang KT, Klöppel G, et al. Intraductal tubulopapillary neoplasms of the bile ducts: clinicopathologic, immunohistochemical, and molecular analysis of 20 cases. Mod Pathol 2015;28:1249-64. [Crossref] [PubMed]
- Jang KT, Park SM, Basturk O, et al. Clinicopathologic characteristics of 29 invasive carcinomas arising in 178 pancreatic mucinous cystic neoplasms with ovarian-type stroma: implications for management and prognosis. Am J Surg Pathol 2015;39:179-87. [Crossref] [PubMed]
- Basturk O, Yang Z, Tang LH, et al. The High-grade (WHO G3) Pancreatic Neuroendocrine Tumor Category Is Morphologically and Biologically Heterogenous and Includes Both Well Differentiated and Poorly Differentiated Neoplasms. Am J Surg Pathol 2015;39:683-90. [Crossref] [PubMed]
- Basturk O, Tang L, Hruban RH, et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol 2014;38:437-47. [Crossref] [PubMed]
- Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg 2000;190:432-45. [Crossref] [PubMed]
- Oberg KE. The management of neuroendocrine tumours: current and future medical therapy options. Clin Oncol (R Coll Radiol) 2012;24:282-93. [Crossref] [PubMed]
- Zhou C, Zhang J, Zheng Y, et al. Pancreatic neuroendocrine tumors: a comprehensive review. Int J Cancer 2012;131:1013-22. [Crossref] [PubMed]
- Basturk O, Yang Z, Tang LH, et al. Increased (20%) Ki67 proliferation index in morphologically well differentiated pancreatic neuroendocrine tumors (PanNETs) correlates with decreased overall survival. Mod Pathol 2013;26:423A.
- Tang LH, Basturk O, Sue JJ, et al. A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am J Surg Pathol 2016;40:1192-202. [Crossref] [PubMed]
- Tang LH, Untch BR, Reidy DL, et al. Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. Clin Cancer Res 2016;22:1011-7. [Crossref] [PubMed]
- Holen KD, Klimstra DS, Hummer A, et al. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol 2002;20:4673-8. [Crossref] [PubMed]
- La Rosa S, Adsay V, Albarello L, et al. Clinicopathologic study of 62 acinar cell carcinomas of the pancreas: insights into the morphology and immunophenotype and search for prognostic markers. Am J Surg Pathol 2012;36:1782-95. [Crossref] [PubMed]
- La Rosa S, Sessa F, Capella C. Acinar Cell Carcinoma of the Pancreas: Overview of Clinicopathologic Features and Insights into the Molecular Pathology. Front Med (Lausanne) 2015;2:41. [Crossref] [PubMed]
- Jäkel C, Bergmann F, Toth R, et al. Genome-wide genetic and epigenetic analyses of pancreatic acinar cell carcinomas reveal aberrations in genome stability. Nat Commun 2017;8:1323. [Crossref] [PubMed]
- Wisnoski NC, Townsend CM Jr, Nealon WH, et al. 672 patients with acinar cell carcinoma of the pancreas: a population-based comparison to pancreatic adenocarcinoma. Surgery 2008;144:141-8. [Crossref] [PubMed]
- Matos JM, Schmidt CM, Turrini O, et al. Pancreatic acinar cell carcinoma: a multi-institutional study. J Gastrointest Surg 2009;13:1495-502. [Crossref] [PubMed]
- Hoorens A, Gebhard F, Kraft K, et al. Pancreatoblastoma in an adult: its separation from acinar cell carcinoma. Virchows Arch 1994;424:485-90. [Crossref] [PubMed]
- Klimstra DS, Wenig BM, Adair CF, et al. Pancreatoblastoma. A clinicopathologic study and review of the literature. Am J Surg Pathol 1995;19:1371-89. [Crossref] [PubMed]
- Abraham SC, Wu TT, Klimstra DS, et al. Distinctive molecular genetic alterations in sporadic and familial adenomatous polyposis-associated pancreatoblastomas: frequent alterations in the APC/beta-catenin pathway and chromosome 11p. Am J Pathol 2001;159:1619-27. [Crossref] [PubMed]
- Tanaka Y, Kato K, Notohara K, et al. Significance of aberrant (cytoplasmic/nuclear) expression of beta-catenin in pancreatoblastoma. J Pathol 2003;199:185-90. [Crossref] [PubMed]
- Hackeng WM, Hruban RH, Offerhaus GJ, et al. Surgical and molecular pathology of pancreatic neoplasms. Diagn Pathol 2016;11:47. [Crossref] [PubMed]
- Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg 2005;200:965-72. [Crossref] [PubMed]
- Tanaka Y, Kato K, Notohara K, et al. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res 2001;61:8401-4. [PubMed]
- Abraham SC, Klimstra DS, Wilentz RE, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol 2002;160:1361-9. [Crossref] [PubMed]
- Estrella JS, Li L, Rashid A, et al. Solid pseudopapillary neoplasm of the pancreas: clinicopathologic and survival analyses of 64 cases from a single institution. Am J Surg Pathol 2014;38:147-57. [Crossref] [PubMed]
- Adsay NV, Basturk O, Saka B, et al. Whipple made simple for surgical pathologists: orientation, dissection, and sampling of pancreaticoduodenectomy specimens for a more practical and accurate evaluation of pancreatic, distal common bile duct, and ampullary tumors. Am J Surg Pathol 2014;38:480-93. [Crossref] [PubMed]
- Verbeke CS, Menon KV. Redefining resection margin status in pancreatic cancer. HPB (Oxford) 2009;11:282-9. [Crossref] [PubMed]
- Adsay NV, Andea A, Basturk O, et al. Secondary tumors of the pancreas: an analysis of a surgical and autopsy database and review of the literature. Virchows Arch 2004;444:527-35. [Crossref] [PubMed]
- Hiotis SP, Klimstra DS, Conlon KC, et al. Results after pancreatic resection for metastatic lesions. Ann Surg Oncol 2002;9:675-9. [Crossref] [PubMed]
- Yuasa T, Inoshita N, Saiura A, et al. Clinical outcome of patients with pancreatic metastases from renal cell cancer. BMC Cancer 2015;15:46. [Crossref] [PubMed]
- Daum O, Klecka J, Ferda J, et al. Gastrointestinal stromal tumor of the pancreas: case report with documentation of KIT gene mutation. Virchows Arch 2005;446:470-2. [Crossref] [PubMed]
- Komoda H, Nishida T, Yumiba T, et al. Primary leiomyosarcoma of the pancreas--a case report and case review. Virchows Arch 2002;440:334-7. [Crossref] [PubMed]
- Zhang H, Yu S, Wang W, et al. Primary mesenchymal tumors of the pancreas in a single center over 15 years. Oncol Lett 2016;12:4027-34. [PubMed]
- Adsay NV, Basturk O, Klimstra DS, et al. Pancreatic pseudotumors: non-neoplastic solid lesions of the pancreas that clinically mimic pancreas cancer. Semin Diagn Pathol 2004;21:260-7. [Crossref] [PubMed]
- Muraki T, Kim GE, Reid MD, et al. Paraduodenal Pancreatitis: Imaging and Pathologic Correlation of 47 Cases Elucidates Distinct Subtypes and the Factors Involved in its Etiopathogenesis. Am J Surg Pathol 2017;41:1347-63. [Crossref] [PubMed]
- Klimstra DS, Conlon KC, Adsay NV. Lymphoplasmacytic sclerosing pancreatitis with pseudotumor formation. Pathol Case Rev 2001;6:94-9. [Crossref]


