Imaging for pancreatic ductal adenocarcinoma
Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains one of the deadliest cancers worldwide. In the U.S. and Europe, it is the fourth leading cause of cancer-related death in both men and women, with an incidence rate similar to its mortality rate (1,2). Tumor aggressiveness and delayed diagnosis are the main reasons for the poor survival rate.
Patients with PDAC are usually asymptomatic in the early phases such that imaging is infrequently obtained in these patients. Once more common symptoms arise, such as jaundice, abdominal pain, weight loss and diabetes (1), patients often undergo cross-sectional imaging allowing the radiologist to make a diagnosis. While complete surgical resection is sought as the only potentially curative treatment for patients with PDAC, unfortunately imaging often confirms unresectable disease, with fewer than 20% of patients having potentially resectable tumor at the time of the diagnosis.
In this review, we will discuss how imaging studies play a central role in the detection and staging of patients with PDAC, enabling the multidisciplinary team to choose the best treatment options for each patient.
Staging
High quality cross-sectional imaging designed for pancreatic evaluation is recommended at presentation prior to stenting, whenever possible, in order to avoid imaging artifacts caused by the stent and complications in the liver such as cholangitis and abscesses that may mimic metastatic disease.
The National Comprehensive Cancer Network (NCCN) recommends that the staging of PDAC should be based on presurgical imaging findings with a classification of the disease as (I) resectable; (II) borderline resectable; (III) locally advanced unresectable; or (IV) disseminated or metastatic (1). Table 1 summarizes how resectability is assessed with respect to vascular involvement (Table 1).
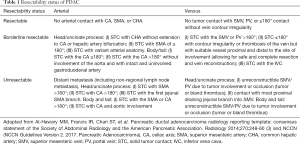
Full table
The following characteristics are evaluated by the radiologist:
- A morphologic evaluation is performed to describe: (i) the appearance of the tumor in the pancreatic parenchymal phase; (ii) its size (maximal axial dimension); (iii) its location (head/uncinate process or body/tail); (iv) the presence of pancreatic duct narrowing or abrupt cutoff with or without upstream dilatation; and (v) whether there is biliary tree abrupt cutoff with or without upstream dilatation. While morphologic evaluation does not directly influence resectability, it will inform the surgeon about the potential surgical approach, i.e., Whipple surgery versus distal pancreatectomy;
- An arterial evaluation includes whether the tumor contacts the (i) superior mesenteric artery (SMA); (ii) celiac axis; (iii) common hepatic artery (CHA); and whether there is any (iv) variant anatomy. These arteries need to be assessed for the degree of solid versus hazy soft-tissue contact, and focal vessel narrowing or contour irregularity. When the SMA is involved it is also important to evaluate the extension to its first branching point. With regards to the CHA, it is also relevant to assess whether the tumor extends to the celiac axis or to the bifurcation of right or left hepatic arteries;
- A venous evaluation includes whether the tumor contacts the (i) main portal vein or (ii) superior mesenteric vein (SMV); whether there is (iii) thrombus within the vein; differentiating between tumor and bland, and whether there are (iv) venous collaterals. The veins are evaluated for the degree of solid versus hazy soft-tissue contact and for focal vessel narrowing/contour irregularity, which can appear as tethering or a tear drop shape;
- The extrapancreatic evaluation consists of comments on the presence and suspicious nature of: (i) liver lesions; (ii) peritoneal nodules; (iii) ascites; (iv) lymph nodes and their location; and (v) other potential extrapancreatic metastasis, such as bones metastases.
In 2014, the Society of Abdominal Radiologists and American Pancreatic Association (APA) collaborated on a reporting template and a lexicon terminology to describe PDAC on computed tomography (CT) and magnetic resonance imaging (MRI) (3). Use of a standardized radiology staging report template is highly recommended to guarantee complete assessment of PDAC and improve communication between radiologists and other specialists on the multidisciplinary team, in order to ensure an optimal staging and overall improve the decision-making process.
Abdominal ultrasound
Overview
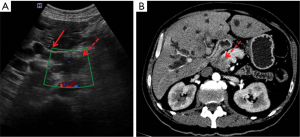
Abdominal ultrasound (US) is a low-cost and non-invasive diagnostic modality that does not produce ionizing radiation and is frequently the first imaging modality requested for patients with abdominal pain and jaundice. It is an operator-dependent modality that does not allow a panoramic view of the abdominal cavity and of the entire pancreas in some patients, mainly due to gas interposition and patient’s body habitus. Thus, the detection of PDAC remains challenging with US. Contrast-enhanced US (CEUS) is known to enhance the accuracy of conventional US by allowing better delineation of the pancreatic mass (4) and characterization of liver metastases (5). Unfortunately in a patient with clinical suspicion of pancreatic disease, a normal US often necessitates additional cross-sectional imaging (CT or MRI) for further evaluation. Even if US detects a pancreatic lesion, CT and MRI remain indicated for staging (Figure 1).
Imaging features
PDAC appears on US as a solid hypoechoic mass with an ill-defined contour, often with upstream dilatation of the pancreatic duct. Lesions located in the head of the pancreas could also cause dilatation of the biliary ducts (Figure 1A). On Doppler, the lesion demonstrates low vascularity and is hypovascular on CEUS.
Liver metastases are hypoechoic and hypovascular on CEUS, but could also demonstrate a rim enhancement in the early phase.
Diagnostic performance
The sensitivity of US in detecting PDAC varies among centers from around 50% to 90% (6,7), probably due to different levels of expertise of the operators and the different stages of disease included in such studies. CEUS may improve the accuracy of US in detecting liver metastases, reaching sensitivity and specificity that ranges from 80% to 95% (8,9).
Multi-detector CT
Overview
Multi-detector computed tomography (MDCT) is a widely available imaging modality with high spatial and temporal resolution, and provides a wide anatomic coverage. It is the most often used modality in the diagnosis and staging of PDAC, allowing quick and comprehensive local and distant staging.
Pancreas protocol
CT should be performed in a helical scanner, preferably in a 64- or greater multidetector row scanner, with a reconstructed slice thickness of at most 3 mm and no gap. With submillimeter slice acquisitions, nearly isotropic voxels are obtained which allows for multiplanar reformats in the coronal and sagittal planes, as well as maximum intensity projection (MIP) and 3D volumetric images used to optimize vascular assessment (1,3).
A neutral oral contrast agent (such as water) is recommended, as well as intravenous iodinated contrast agent injected at a rate of 3 to 5 mL/sec. The following dynamic phases are mandatory: (I) pancreatic parenchymal phase (40 to 50 sec) and (II) portal venous phase (65 to 70 sec). In the first phase, the pancreatic parenchyma reaches maximal enhancement and the arteries are adequately opacified, while in the portal venous phase, the portomesenteric veins are opacified as well as the liver parenchyma are opacified (1,3).
This dual-phase pancreatic protocol allows an adequate morphologic, arterial, venous and abdominal extrapancreatic evaluation, which is essential for staging. Inclusion of the chest and pelvis allows for more comprehensive staging.
Imaging features
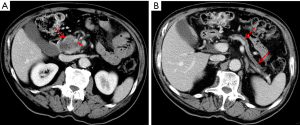
PDAC is classically visible as an ill-defined hypoattenuating mass that appears hypovascular compared with the background parenchyma (Figures 1B,2A), with gradual enhancement in the portal venous phase. However, 5% to 27% of PDAC show similar attenuation and enhancement as the normal parenchyma and can be occult on CT, mainly in cases where tumors are smaller than 2 cm (10-12). In such instances, it is important to look for secondary signs of malignancy, such as abrupt cutoff of the pancreatic duct with upstream dilatation, mass effect, abnormality of the pancreatic contour and less fat displacement than the remaining parenchyma (Figures 2B,3).
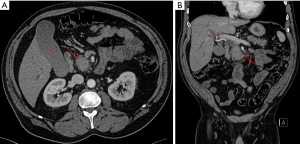
Liver metastases are usually hypovascular and demonstrate poorly defined margins and rim enhancement (Figure 4). With regards to lymph node involvement, the imaging features that are suspicious for malignancy are short axis longer than 1 cm, abnormal morphology, heterogeneity or central necrosis (3). Other sites of metastatic disease include the peritoneum and mesentery, lungs, and bones (Figure 5).
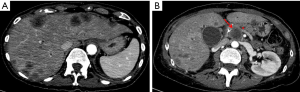
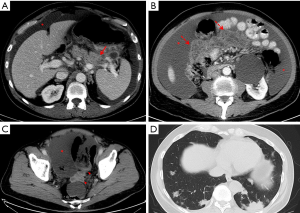
Diagnostic performance
The sensitivity of MDCT for detection of pancreatic malignancy can be as high as 89% to 97% (13). However, for lesions smaller than 1.5 cm the sensitivity has been reported to be as low as 67% (14).
MRI
Overview
MRI has rapidly evolved since the turn of the millennium, improving in imaging quality and acquisition time. MRI has higher soft tissue contrast resolution but generally it is not superior to CT in detection and staging of PDAC (15). However, some studies have demonstrated that MRI can increase lesion conspicuity and be helpful in characterizing smaller and isoattenuating tumors on CT, and distinguish focal fatty infiltration in the pancreatic head (15,16). MRI can also add value in the detection and characterization of liver metastases when CT findings are equivocal. Magnetic resonance cholangiopancreatography (MRCP) is included in an MRI examination of the pancreas in order to improve ductal evaluation and for delineating stones (6).
Barriers to MRI include its high cost, lower availability compared with CT, and potential contraindications related to MRI contrast media and certain medical implants. The use of MRI in PDAC is also considered challenging due to artifacts from respiratory motion or artifacts related to metal stent. Furthermore, MRI has slightly lower spatial resolution than CT which may reduce the imaging quality for vascular assessment.
Considering these practical constraints, the use of MRI over CT depends upon availability, local expertise and a patient’s contraindications to CT iodinated contrast. Usually, MRI is a problem-solving tool to evaluate suspected pancreatic tumors not visible on CT, offer as an alternative to CT when iodinated contrast is less safe, and characterize indeterminate liver lesions.
Pancreas protocol
The following sequences are recommended by the NCCN in the MRI pancreatic adenocarcinoma protocol: (I) T2-weighted imaging (WI) single-shot fast spin-echo (SSFSE) in coronal ± axial with slice thickness smaller than 6 mm; (II) T1WI in-phase and out-phase gradient echo (GRE) in the axial plane with slice thickness smaller than 6 mm; (III) T2WI fat-suppressed fast spin-echo (FSE) in the axial plane with slice thickness smaller than 6 mm; (IV) diffusion-weighted imaging (DWI) in the axial plane with slice thickness smaller than 6 mm; (V) pre and dynamic post intravenous contrast administration 3D T1WI fat-suppressed GRE in the pancreatic, portal venous and equilibrium phases, in the axial plane, with slice thickness as thin as possible (2 to 3 mm or 4 to 6 mm if overlapping); and (VI) T2WI MRCP, preferably 3D volumetric acquisition in the coronal plane and slice thickness smaller than 3 mm (1).
Imaging features
The pancreas on MRI is normally very high in signal intensity (SI) on T1WI with intense and homogeneous enhancement in the arterial pancreatic phase. PDAC demonstrates low SI on T1WI, shows restricted diffusion and is hypovascular with progressive delayed enhancement on post-contrast phases. The tumor usually causes obstruction of the pancreatic and biliary duct when in the head. Atrophic pancreas upstream of the tumor typically shows low SI on T1WI. Ductal dilatation and abrupt cutoff are easily demonstrated on T2WI sequences (Figure 6A), including MRCP (Figure 6B).
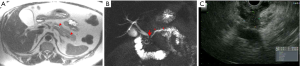
The imaging features of extrapancreatic disease were previously described on CT and appear similar on postcontrast MR images.
Diagnostic performance
The sensitivity and specificity of MRI for detection of PDAC is similar to CT (17). With regards to detection of liver metastases, MRI shows increased sensitivity and overall accuracy, particularly with the use of a DWI sequence and hepatobiliary-specific contrast agent.
Endoscopic ultrasound (EUS)
Overview
EUS is a safe and effective method for imaging of the pancreas that is useful in the diagnosis and evaluation of PDAC in conjunction with CT or MRI. EUS produces high-resolution images of the pancreas and under endoscopic guidance, fine needle aspiration or fine needle biopsy can be performed to obtain diagnostic sampling of the mass once visualized in the pancreas. One other potential advantage of EUS is that there is no need to use intravenous contrast, which is particularly useful for patients with contraindications to CT or MRI. With EUS, the most important limitation is the inability to stage the disease beyond the pancreas. NCCN guidelines state that EUS is not recommended as a routine staging tool but in select cases may be complementary to CT for staging (1). For example, EUS may be complementary to CT in patients whose initial scan shows no or an equivocal lesion, or when there is uncertainty regarding vascular involvement. Furthermore, with EUS, one has the ability to perform diagnostic sampling with low risk.
Pancreas protocol
EUS is an endoscopic approach to imaging, thus the patient is generally provided with sedation (conscious sedation or monitored anesthesia care). Once sedated, an endoscope with an US probe on the tip is advanced into the stomach or the duodenum and used to image the pancreas through the gastric or intestinal wall. Views of the pancreas head and neck can be obtained from the duodenum. Views of the pancreas body and tail can be obtained from the stomach.
Imaging features
Pancreas masses can be well visualized and appear as round or irregular hypoechoic lesions within the pancreas, often with upstream dilatation of the pancreatic duct. EUS is often successful at detecting small masses less than 2 cm that may not be seen on CT or MRI (Figure 6C).
Diagnostic performance
EUS has being shown to be one of the most accurate imaging modalities for the detection of pancreatic focal lesions, especially in patients with small tumors less than 2 to 3 cm. The sensitivity for the detection of pancreatic masses is reported to be as high as 93–100% (18). EUS is able to provide accurate information on staging of pancreas adenocarcinoma with the ability to detect vascular involvement; however, studies assessing the sensitivity and specificity of EUS compared with CT have a wide range of results. As MDCT has significantly improved over time, the sensitivity and specificity of MDCT has paralleled that of EUS with some additional advantages. EUS, while accurate for staging and determining resectability, has a few drawbacks including decreased sensitivity of vascular invasion with small lesion of the pancreas, dependence on expertise of the operator, and the need for sedation (19).
Positron emission tomography (PET)/CT
Overview
PET/CT is an imaging modality that combines functional imaging from PET with anatomical images from CT, both acquired in a single gantry covering the whole body. The most common radiotracer on PET is 18F-flurodeoxyglucose (18F-FDG), which is a glucose analogue. PDAC is generally associated with an overexpression of glucose transporter 1, resulting in increased 18F-FDG uptake on PET-CT (20). The wide anatomical coverage of PET-CT allows the evaluation of the entire body which is helpful for evaluation of metastatic disease. A main limitation of this imaging modality is the low spatial resolution and possibility of false-positive uptake in normal structures or benign diseases, such as inflammatory processes (21).
Imaging features
PDAC as well as metastatic disease appear as an area of increased uptake of 18F-FDG; however, depending on the tumor aggressiveness and degree of desmoplastic reaction, there can be low or no 18F-FDG uptake (Figure 7).
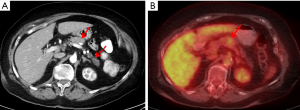
Diagnostic performance
Studies have demonstrated sensitivity ranging from 85% to 95% and specificity of 61% to 94% (22) in the detection of PDAC. With regards to lymph node, its sensitivity is low ranging from 21% to 42% (23,24). PET/CT is potentially advantageous in M staging with high sensitivity for the detection of lung, liver, peritoneal and bone metastases (23,25). Regarding local staging of PDAC, the role of PET/CT is limited without the use of intravenous contrast media on CT.
Tumor response to chemotherapy and radiation therapy can also be assessed by PET-CT, with studies demonstrating a decrease in standardized uptake value (SUV) during or following therapy, with the potential benefit of being able to adjust therapy accordingly (26-28). Whether 18F-FDG uptake in the primary neoplasm predicts prognosis is debatable, with some studies suggesting SUV to be an independent predictor of survival (29,30) and others indicating that baseline SUV is not predictive of disease-free or overall survival (31).
Currently, PET/CT is considered as an adjunct modality to CT in the evaluation in high-risk patients, including borderline resectable disease, markedly elevated CA 19.9, large primary tumors or large regional lymph nodes (1).
Table 2 summarizes the imaging modalities in the staging of PDAC.
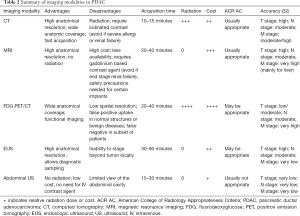
Full table
Future directions
Some novel techniques are being used in the detection and staging of PDAC. Dual energy CT has shown good results in improving contrast-to-noise which enhance the detection of small tumors. Dual energy CT can also reduce metal artifacts, which can help in the staging of patients with stent (33). Furthermore, PET-MRI has demonstrated promising results by combining high anatomical contrast from MRI and metabolic information from PET, improving the detection of small lesions and with no radiation (34).
Conclusions
Imaging plays a central role in the detection and local and distant staging of PDAC, particularly CT and MRI. Thus, imaging is crucial in multidisciplinary team efforts to choose the best treatment option. In cases of resectable and borderline resectable disease, imaging provides the surgeon with a roadmap and in cases of unresectable disease, imaging reduces morbidity from unnecessary surgery.
Acknowledgements
We thank Joanne Chin for editorial assistance, Brunna de Oliveira and Camila Tavares for assistance with figures.
Funding: This paper was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- NCCN. NCCN Guidelines Version 3. 2017 Pancreatic Adenocarcinoma. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
- Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v56-68. [Crossref] [PubMed]
- Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014;270:248-60. [Crossref] [PubMed]
- Oshikawa O, Tanaka S, Ioka T, et al. Dynamic sonography of pancreatic tumors: comparison with dynamic CT. AJR Am J Roentgenol 2002;178:1133-7. [Crossref] [PubMed]
- D'Onofrio M, Crosara S, De Robertis R, et al. Contrast-Enhanced Ultrasound of Focal Liver Lesions. AJR Am J Roentgenol 2015;205:W56-66. [Crossref] [PubMed]
- Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol 2014;20:7864-77. [Crossref] [PubMed]
- Rickes S, Unkrodt K, Neye H, et al. Differentiation of pancreatic tumours by conventional ultrasound, unenhanced and echo-enhanced power Doppler sonography. Scand J Gastroenterol 2002;37:1313-20. [Crossref] [PubMed]
- Correas JM, Low G, Needleman L, et al. Contrast enhanced ultrasound in the detection of liver metastases: a prospective multi-centre dose testing study using a perfluorobutane microbubble contrast agent (NC100100). Eur Radiol 2011;21:1739-46. [Crossref] [PubMed]
- Dietrich CF, Kratzer W, Strobe D, et al. Assessment of metastatic liver disease in patients with primary extrahepatic tumors by contrast-enhanced sonography versus CT and MRI. World J Gastroenterol 2006;12:1699-705. [Crossref] [PubMed]
- Prokesch RW, Chow LC, Beaulieu CF, et al. Isoattenuating pancreatic adenocarcinoma at multi-detector row CT: secondary signs. Radiology 2002;224:764-8. [Crossref] [PubMed]
- Kim JH, Park SH, Yu ES, et al. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology 2010;257:87-96. [Crossref] [PubMed]
- Yoon SH, Lee JM, Cho JY, et al. Small (≤20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology 2011;259:442-52. [Crossref] [PubMed]
- Wong JC, Lu DS. Staging of pancreatic adenocarcinoma by imaging studies. Clin Gastroenterol Hepatol 2008;6:1301-8. [Crossref] [PubMed]
- Legmann P, Vignaux O, Dousset B, et al. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR Am J Roentgenol 1998;170:1315-22. [Crossref] [PubMed]
- Park HS, Lee JM, Choi HK, et al. Preoperative evaluation of pancreatic cancer: comparison of gadolinium-enhanced dynamic MRI with MR cholangiopancreatography versus MDCT. J Magn Reson Imaging 2009;30:586-95. [Crossref] [PubMed]
- Schima W, Függer R. Evaluation of focal pancreatic masses: comparison of mangafodipir-enhanced MR imaging and contrast-enhanced helical CT. Eur Radiol 2002;12:2998-3008. [PubMed]
- Koelblinger C, Ba-Ssalamah A, Goetzinger P, et al. Gadobenate dimeglumine-enhanced 3.0-T MR imaging versus multiphasic 64-detector row CT: prospective evaluation in patients suspected of having pancreatic cancer. Radiology 2011;259:757-66. [Crossref] [PubMed]
- Săftoiu A, Vilmann P. Role of endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. J Clin Ultrasound 2009;37:1-17. [Crossref] [PubMed]
- Tamburrino D, Riviere D, Yaghoobi M, et al. Diagnostic accuracy of different imaging modalities following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst Rev 2016;9:CD011515. [PubMed]
- Reske SN, Grillenberger KG, Glatting G, et al. Overexpression of glucose transporter 1 and increased FDG uptake in pancreatic carcinoma. J Nucl Med 1997;38:1344-8. [PubMed]
- Diederichs CG, Staib L, Vogel J, et al. Values and limitations of 18F-fluorodeoxyglucose-positron-emission tomography with preoperative evaluation of patients with pancreatic masses. Pancreas 2000;20:109-16. [Crossref] [PubMed]
- Wang XY, Yang F, Jin C, et al. Utility of PET/CT in diagnosis, staging, assessment of resectability and metabolic response of pancreatic cancer. World J Gastroenterol 2014;20:15580-9. [Crossref] [PubMed]
- Kauhanen SP, Komar G, Seppänen MP, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg 2009;250:957-63. [Crossref] [PubMed]
- Heinrich S, Goerres GW, Schäfer M, et al. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg 2005;242:235-43. [Crossref] [PubMed]
- Asagi A, Ohta K, Nasu J, et al. Utility of contrast-enhanced FDG-PET/CT in the clinical management of pancreatic cancer: impact on diagnosis, staging, evaluation of treatment response, and detection of recurrence. Pancreas 2013;42:11-9. [Crossref] [PubMed]
- Chang ST, Goodman KA, Yang GP, et al. Stereotactic body radiotherapy for unresectable pancreatic cancer. Front Radiat Ther Oncol 2007;40:386-94. [Crossref] [PubMed]
- Yoshioka M, Sato T, Furuya T, et al. Role of positron emission tomography with 2-deoxy-2-[18F]fluoro-D-glucose in evaluating the effects of arterial infusion chemotherapy and radiotherapy on pancreatic cancer. J Gastroenterol 2004;39:50-5. [Crossref] [PubMed]
- Kittaka H, Takahashi H, Ohigashi H, et al. Role of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in predicting the pathologic response to preoperative chemoradiation therapy in patients with resectable T3 pancreatic cancer. World J Surg 2013;37:169-78. [Crossref] [PubMed]
- Sperti C, Pasquali C, Chierichetti F, et al. 18-Fluorodeoxyglucose positron emission tomography in predicting survival of patients with pancreatic carcinoma. J Gastrointest Surg 2003;7:953-9; discussion 9-60. [Crossref] [PubMed]
- Zimny M, Fass J, Bares R, et al. Fluorodeoxyglucose positron emission tomography and the prognosis of pancreatic carcinoma. Scand J Gastroenterol 2000;35:883-8. [Crossref] [PubMed]
- Heinrich S, Schafer M, Weber A, et al. Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity: results of a prospective phase II trial. Ann Surg 2008;248:1014-22. [Crossref] [PubMed]
- Qayyum A, Tamm EP, Kamel IR, et al. ACR Appropriateness Criteria(®) Staging of Pancreatic Ductal Adenocarcinoma. J Am Coll Radiol 2017;14:S560-S9. [Crossref] [PubMed]
- Frellesen C, Fessler F, Hardie AD, et al. Dual-energy CT of the pancreas: improved carcinoma-to-pancreas contrast with a noise-optimized monoenergetic reconstruction algorithm. Eur J Radiol 2015;84:2052-8. [Crossref] [PubMed]
- Jha P, Bijan B, Melendres G, et al. Hybrid imaging for pancreatic malignancy: clinical applications, merits, limitations, and pitfalls. Clin Nucl Med 2015;40:206-13. [Crossref] [PubMed]


