Practical genetics of colorectal cancer
Overview
The estimated incidence of colorectal cancer (CRC) in the United States during 2013 is 142,820, with an estimated mortality of 50,830 (1). Worldwide estimates for 2008 are an incidence of 1,234,000 and mortality of approximately 608,000 (2). Approximately 3% (1 of every 35 cases) of CRC is attributable to Lynch syndrome (LS), the most common hereditary syndrome predisposing to CRC (3). Polyposis syndromes such as familial adenomatous polyposis (FAP) contribute a lesser percentage of the total CRC burden. Figure 1 demonstrates the heterogeneity of identified hereditary CRC syndromes.
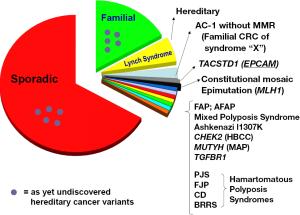
Familial CRC, meaning one or more (and, by some definitions, two or more) first-degree and/or second-degree relatives of the index case manifesting CRC, indicates that the index case has an approximate 2-fold increased CRC risk. Familial CRC by this definition constitutes about 20% of the total CRC incident burden.
Lynch syndrome
Mismatch repair mutations
LS is inherited in an autosomal dominant pattern. Mutations in mismatch repair (MMR) genes have been identified in LS patients (4): MLH1 (5), located on chromosome 3p21.3; MSH2 (6,7) and MSH6 (8), both located on 2p21; and PMS2 (9), located on 7p22;. Approximately 90% of the identified LS mutations involve MLH1 or MSH2, while mutations in the MSH6 gene account for approximately 10%.
Individuals carrying germline mutations of MLH1 or MSH2 have a lifetime risk for CRC on the order of 80% (10). Seventy percent of the CRCs arise proximal to the splenic flexure. The average age at diagnosis is 45 years, compared with 63 years in the general population. Multiple synchronous and metachronous CRCs are seen in the syndrome, with 30% of patients developing a second cancer within 10 years if a limited operation (right hemicolectomy or segmental resection) is done for the initial cancer as opposed to a subtotal colectomy should metastasis be absent. Even when total abdominal colectomy is performed, the rectum is still at risk; Rodriguez-Bigas et al. (11) reported that 12% of LS patients had rectal cancer within 12 years after colectomy.
Pathology of CRCs is more often poorly differentiated in LS, with an excess of mucoid and signet-cell features, a Crohn’s-like reaction, and a significant excess of tumor-infiltrating lymphocytes. Increased survival from CRC also occurs in the syndrome (12-14).
Extracolonic cancers
Extracolonic cancers are common in LS (15). Endometrial carcinoma is the most notable, women with LS having a 20% to 60% lifetime risk for endometrial cancer (10,16). There is also an increased risk for carcinoma of the stomach, ovary, renal pelvis and ureter, small bowel, hepatobiliary tract, and pancreas. Glioblastoma multiforme is seen in the Turcot’s syndrome variant (17). Benign and malignant sebaceous skin tumors occur in combination with CRC and other internal malignancies in the Muir-Torre syndrome variant (18). Quite recently, molecular genetic evidence has demonstrated that breast and prostate cancer in LS mutation carriers may not be spontaneously developing cancers, but rather are part of the LS tumor spectrum (19).
Genotype-phenotype heterogeneity
LS, not unlike other autosomal dominantly inherited disorders, is noteworthy for genotypic and phenotypic heterogeneity (20-22). MSH2 mutations may predispose to an excess of extracolonic cancer compared with its MSH1 counterpart. In a study that included 138 families with LS wherein mutations were identified in 79 of these families (34 with MLH1, 40 with MSH2, 5 with MSH6), Vasen et al. (23) found that the lifetime risk for developing cancer at any anatomic site was significantly higher for MSH2 mutation carriers when compared with MLH1 mutation carriers (P<0.01). With respect to specific anatomic sites, MSH2 mutation carriers in this study were found to have a significantly higher risk of developing cancer of the urinary tract (P<0.05).
Carriers of MSH6 germline mutations have been found to harbor a lower risk for CRC than carriers of MSH2 and MLH1 mutations. However, women with an MSH6 mutation are at a higher risk for endometrial cancer than those carrying one of the other mutations. In a study involving mutation analysis of 20 families with an MSH6 germline mutation, Hendriks et al. (24) compared the cancer risks between MSH6 and MLH1/MSH2 mutation carriers. They identified a total of 146 MSH6 mutation carriers, among whom the cumulative risk for CRC was 69% for men and only 30% for women. Endometrial carcinoma was present in 71% of the women by age 70. When all LS-related tumors were considered, the risk was significantly lower in MSH6 mutation carriers compared with MLH1 or MSH2 mutation carriers (P=0.002). Among females with MSH6 mutations, the risk of CRC was significantly lower (P=0.0049) but the risk for endometrial cancer significantly higher (P=0.02) than in MLH1 and MSH2 mutation carriers.
Childhood cancers and other unusual tumors
Although childhood and hematological cancers are not generally considered to be part of the LS tumor spectrum, Bandipalliam (25), de Vos and colleagues (26), and Scott and colleagues (27) have described a syndrome characterized by a biallelic inactivation of the MMR genes, leading to a recessive MMR-deficiency syndrome predisposing to “..childhood malignancies such as lymphoma, leukemia, brain and gastrointestinal tumors and features of neurofibromatosis type 1” (28). Menko and colleagues (29) have also noted that there has been recent evidence of homozygous mutations in the MMR genes MLH1, MSH2, and PMS2 in children with hematological malignancies, solid tumors, and clinical signs (multiple café-au-lait spots) of neurofibromatosis type 1 (30-37), and have described a child with multiple café-au-lait spots in association with an oligodendroglioma at age 10, followed by rectal carcinoma at age 12, which lesions were ascribed to a homozygous MSH6 mutation with predicted pathogenic effects.
Molecular pathogenesis of tumors outside the usual LS cancer spectrum remains controversial. Broaddus and colleagues (38) described two young MSH2 mutation carriers, one age 34 who developed an adrenal cortical carcinoma, and the second a 39-year-old woman who had a diagnosis of anaplastic carcinoma of the thyroid, tumors not usually associated with LS. Berends and colleagues (39) reported a female with an MSH2 germline mutation, ovarian cancer, and three metachronous CRCs who was also found to have an adrenal cortical carcinoma. It is of further interest that the original proband in Lynch’s Family N, reported in 1966 (40), manifested adrenal cortical carcinoma. Other reports of rare tumors in Lynch syndrome include a malignant fibrous histiocytoma arising in a patient with a germline MSH2 mutation and a positive LS family history (41), and a male with a germline MLH1 mutation and a positive LS family history who developed infiltrating ductal carcinoma of the breast 30 years after early-onset CRC (42). The dilemma remains as to whether these tumors are related to defects in DNA MMR, or whether they have arisen independently of this MMR defect.
EPCAM and LS
The underlying molecular defect responsible for a portion of families manifesting a LS phenotype without an identified MMR mutation, is a mutation in the epithelial cell adhesion molecule (EPCAM) gene, located immediately 5' of MSH2 (43-45). A germline deletion removing the 3' end of EPCAM results in tissue-specific methylation and silencing of the otherwise intact MSH2 gene.
Ligtenberg et al. (45) were the first to describe such families. Tumors from individuals with EPCAM mutations had high microsatellite instability (MSI) and immunohistochemistry (IHC) showed a loss of MSH2 protein even though there was no identifiable mutation of the MSH2 gene (43,45). In approximately 19% of LS-consonant families that lacked MLH1/MSH2 mutations the EPCAM mutations co-segregated with the LS phenotype (43). In a study by Kuiper et al. (46), EPCAM deletions were found to account for about 2.3% of explained MSH2-deficient families.
Expression of the EPCAM gene is restricted primarily to epithelial tissues. This appears to influence the phenotypic expression associated with this type of mutation, which may differ from that of carriers of MSH2 mutations. Notably, cancer expression appears to be almost exclusively limited to the colorectum, with a paucity of the endometrial cancers that would be expected with an MSH2 mutation. It must be noted, however, that when the EPCAM deletion extends into, or is in close proximity to, the MSH2 gene, the risk for endometrial cancer becomes equivalent to those with MSH2 mutations. Determination of the precise boundaries of deletions involving EPCAM is likely to be important for clinical management of these families. CRC occurs in EPCAM deletion carriers with a frequency comparable to that of carriers of an MSH2 mutation.
We have studied a family containing more than 700 individuals (47). An EPCAM deletion was identified in this highly-extended family, which has been studied by us for more than 35 years; 50 members of this family have manifested CRC, providing strong evidence of the potential impact of a single mutation event (47).
Familial colorectal cancer type X
As mentioned earlier, a MMR germline mutation is able to be identified in approximately 60% of families fulfilling clinical criteria for LS. Lindor et al. (48) studied 161 pedigrees from such families divided into those with (group A) vs. those without (group B) MMR deficiency through tumor testing. This involved 3,422 relatives for the analysis. Findings disclosed that group A families showed an increased incidence of LS-related cancers, while group B families showed an increased incidence of only CRC [SIR, 2.3; 95% confidence interval (CI), 1.7-3.0], and to a lesser extent than group A (SIR, 6.1; 95% CI, 5.2-7.2) (P<0.001). These authors indicated that such families should not be described or counseled as LS and suggested a designation of “familial colorectal cancer type X” (48).
Somewhat similar studies (49,50) assessed features of the clinically-defined LS families that lacked MSI. Individuals were found to be older at CRC diagnosis than those with MSI and therein their tumors were less commonly proximal, less often clearly differentiated and mucinous, and more often showed DNA aneuploidy. They did not present as often with multiple cancers and there was a lower incidence of CRC and endometrial cancer.
IHC and MSI
Thanks to molecular genetic advances, the diagnosis of LS can be aided significantly by demonstrating the presence of MSI within the tumor and/or IHC that identifies a loss of MMR proteins. Diagnosis of LS can be verified by the identification of a germline mutation in one of the MMR genes, the sine qua non for LS’s confirmation. Ideally, this molecular testing is performed on high-risk individuals from families that show the clinical-pathology features of LS.
All cells of individuals affected with LS carry a nonfunctioning allele of a DNA MMR gene; if the wild-type allele is lost or inactivated, the cell can no longer repair DNA mismatches that inevitably arise during DNA replication, leading to the phenomenon of MSI, in which cells have varying lengths of a given microsatellite (an area with multiple repeats of one nucleotide or one pair of nucleotides). Therefore, testing for MSI has been used in screening for CRC associated with MMR deficiency. However, it must be noted that 10-15% of sporadic CRCs demonstrate MSI, the vast majority of which are caused by acquired hypermethylation of the MLH1 promoter (51). The presence of a BRAF mutation is a good surrogate for methylation testing to identify non-LS silencing of MLH1.
When one suspects that a tumor has high MSI (MSI-H), IHC stains for the protein product of the DNA MMR genes MLH1 and MSH2 can be used for confirmation. For example, Marcus et al. (52) found that 37 of 38 neoplasms known to be MSI-H showed an absence of MLH1 or MSH2 expression, and 34 of 34 microsatellite stable (MSS) tumors had intact staining.
Molecular genetic screening of all CRC patients
With the ability to identify patients at risk for LS cancers, and given the relative frequency of the syndrome, the question has been raised of whether all cases of CRC should be tested. Hampel et al. (3) investigated 500 consecutive CRC patients and found that 18 (3.6%) had met clinical criteria for LS. All of these patients had CRCs with MSI. LS was correctly diagnosed in 17 (94%) of these 18 cases by IHC. Of interest was the finding that only 8 cases (44%) were diagnosed at less than 50 years and only 13 cases (72%) met the revised Bethesda Guidelines for MSI testing; these authors point out that this means that if only patients fulfilling the Bethesda Guidelines were tested, 28% of LS mutation carriers would not be identified.
The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (53) supported the Hampel et al. findings (3) and concluded that using genetic testing strategies to reduce morbidity and mortality in CRC probands and their relatives at high risk for and/or affected by LS is a sound and prudent cost-effective measure. Using IHC as the preliminary diagnostic test was shown to be the most efficient screening method, since it reduces the need for sequencing all four of the genes normally tested for LS, to sequencing of only the gene(s) indicated by lack of MMR protein.
Ladabaum et al. (54) investigated cost-effectiveness of a Markov model to identify LS with attention to sex, age at screening, and differential effects for probands and relatives. The target population comprised all of those with newly-diagnosed CRC and their relatives. The time horizon was lifetime, and the perspective was third-party payor. The outcome measures were life-years, cancer cases and deaths, costs coupled with incremental cost-effectiveness ratios. The results considered current rates of germline testing, screening, and prophylactic surgery, which “…reduced deaths from colorectal cancer by 7% to 42% and deaths from endometrial and ovarian cancer by 1% to 6%. Among tumor-testing strategies, immunohistochemistry followed by BRAF mutation testing for all persons with CRC was preferred, with an incremental cost-effectiveness ratio of $36,200 per life-year gained.”
The authors concluded that widespread CRC testing led to LS diagnosis yielding substantial benefits at acceptable cost. This was particularly noteworthy for women with an LS mutation, enabling them to begin regular screening and consider the option of risk-reducing gynecologic surgery with the result of cost-effectiveness of this testing, which was dependent on the participation among relatives at risk for LS. In an accompanying editorial (55), Burt noted that the cost-effectiveness of these approaches “…was very sensitive to the number of relatives who would undergo mutation-specific testing after a disease-causing mutation was found in an index case. It is interesting that success of tumor testing first has already led some institutions and health policy organizations to recommend MSI or IHC testing in all patients with colon cancer and perhaps in all women with endometrial cancer to find those who should then have genetic testing for Lynch syndrome (56-58).”
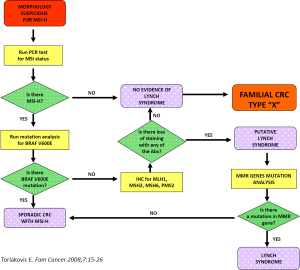
Such an approach to cancer prevention and control may not appear to be practical in most clinical practice settings at the community level. Nevertheless, the rapid development of centers with molecular genetic expertise and the increased availability of genetic counselors, heightened by the ongoing advances in molecular genetics, can potentially provide even more personalized genetic screening services to high-risk families. Figure 2 depicts a possible algorithm for identifying LS and similar disorders.
CRC prevention: colonoscopy screening
There are highly significant clinical implications for diagnosis of LS. Surveillance for CRC in those harboring a mutation is highly effective and considerably less costly than a lack of CRC surveillance (59,60).
Because of the early age of CRC onset in LS and its penchant for the proximal colon, full colonoscopy should be initiated by age 20 to 25 in germline mutation carriers and those at increased cancer risk based on their position in the pedigree. Colonoscopy should be performed at least every one to two years, given the problem of accelerated carcinogenesis of CRC in LS (20,61). We prefer every other year in high-risk patients who have not had DNA testing and annually in patients with LS germline mutations or who are obligate gene mutation carriers.
Järvinen and colleagues (59) showed the benefit of colonoscopic screening in LS through a controlled clinical trial extending over 15 years (59). The incidence of CRC was compared in two cohorts of at-risk members of 22 LS families. CRC developed in eight screened subjects (6%), compared with 19 controls (16%; P=0.014), providing a reduction of 62% in the CRC rate. All CRCs in the screened group were local and caused no deaths, compared with nine deaths caused by CRC in the controls. It was concluded that CRC screening at three-year intervals more than cuts in half the risk of CRC, prevents CRC deaths, and decreases overall mortality by about 65% in LS families. The relatively high incidence of CRC even in the screened subjects (albeit without deaths) argues for shorter screening intervals, a consideration also supported by Vasen et al. (62) who discovered five interval cancers in LS patients within 3-1/2 years following a normal colonoscopy.
In reviewing this subject, Church (63) has suggested that interval CRCs developed from normal epithelium within three years or from adenomas that were missed. It is important to realize that colonoscopy “miss” rates are as high as 29% for polyps <5 mm in diameter (64). Patients should be advised that colonoscopy is not a perfect screening procedure, and the option of prophylactic colectomy should be discussed (65,66).
Surgical measures
Subtotal colectomy as a prophylactic measure among LS patients remains controversial, but patients who carry germline mutations should be offered this option as an alternative to lifetime colonoscopic surveillance. Genetic counseling must be provided so that patients can be in a better position to evaluate the various management strategies. Church (66) and Lynch (65) both suggest that prophylactic surgery should be an option for patients likely to show reduced compliance for colonoscopy.
Syngal and colleagues (67) examined the life expectancy and quality-adjusted life expectancy benefits resulting from endoscopic surveillance and prophylactic colectomy among carriers of germline mutations for LS. Compared with no intervention, both risk-reduction programs showed large gains in life expectancy for mutation carriers, with benefits of 13.5 years for surveillance and 15.6 years for prophylactic proctocolectomy at 25 years of age. The benefits of prophylactic colectomy decreased with increasing age.
Gynecologic screening and surgical management
Women who carry a germline mutation for LS should have annual screening for endometrial cancer beginning at age 30 to 35 years. Endometrial aspiration and transvaginal ultrasound are advised for screening; however, we lack evidence-based data showing survival benefit from such screening. Prophylactic hysterectomy and bilateral salpingo-oophorectomy can be considered when childbearing is completed. Schmeler et al. (68) showed a significant reduction in endometrial and ovarian cancer among those LS patients who underwent prophylactic surgery when compared with those who did not.
LS patients with a family history of kidney cancer and/or hematuria should have annual ultrasound and urinalysis with cytologic examination, beginning at age 30 or at first evidence of hematuria. Evidence-based data showing survival advantage for urologic, gastric, and small bowel screening are not available. Periodic upper endoscopy should be performed in families with gastric or small bowel cancer. Those of Asian origin show a higher frequency of gastric cancer.
Familial adenomatous polyposis
Familial adenomatous polyposis (FAP) is inherited in an autosomal dominant pattern. The incidence is 1 in 6,000 to 1 in 13,000; Powell and colleagues estimated that more than 50,000 families in the United States could benefit from genetic counseling for this disorder (69). Affected individuals carry germline mutations of the APC gene on chromosome 5q21-q22 (70-74). About one-third of FAP patients have no family history and probably represent new mutations. Many mutations of APC have been described; 80% of them are truncating. There is some correlation between the position of the truncating mutation and phenotype (discussed below).
Affected individuals have multiple colonic adenomas and, if untreated, will inevitably develop CRC. Adenomas generally arise in the mid to late teens; 95% of mutation carriers have adenomas by age 35. More than 90% of FAP patients develop duodenal adenomas, but only about 5% of these adenomas develop carcinogenesis. Gastric polyposis is seen in at least 50% of affected patients; most of the polyps are fundic gland polyps, but gastric adenomas do occur. Gastric carcinoma risk, however, is not appreciably elevated in Western FAP patients. In contrast, Japanese and Korean families with FAP have a three- to four-fold excess risk for gastric carcinoma (75).
Extra-intestinal manifestations of FAP include desmoid tumor, hepatoblastoma, thyroid carcinoma, medulloblastoma, and a litany of benign lesions: sebaceous or epidermoid cysts, lipomas, osteomas, supernumerary teeth, congenital hypertrophy of retinal pigment epithelium, and juvenile nasopharyngeal angiofibromas. Brain tumors, particularly medulloblastomas, are a feature of the Turcot’s variant of FAP (17).
The attenuated variant of FAP (AFAP) presents a particularly difficult diagnostic challenge (76-78). Adenomas can be sparse (one or two in some patients, dozens in others) and are often right-sided, both features having the potential of confusion with LS. The adenomas appear at a later age (35-40 years) than in classic FAP, as do colon carcinomas (55 years). Upper gastrointestinal manifestations (fundic gland polyps and duodenal adenomas) are seen with the same, or possibly a greater, frequency as in classic FAP. AFAP families have been described that have mutations near the proximal end of the APC gene as well some that have mutations at the extreme distal end.
Genetic testing is available for FAP. If a mutation is found in an affected family member, other at-risk family members can easily be tested for the mutation. Genetic testing is not recommended for children younger than 10 years of age. If no mutation is found in an affected family member, the test is considered uninformative; a negative result in that setting does not rule out FAP.
Randomized, controlled trials demonstrating the efficacy of screening and management regimens have not been performed. Nevertheless, the following recommendations are generally agreed upon: affected or at-risk individuals should have annual flexible sigmoidoscopy beginning by age 10 to 12. If the family history suggests AFAP, then colonoscopy is required because the adenomas are more likely to be proximal, but the screening can begin later (age 20). Prophylactic colectomy should be considered once multiple adenomas have appeared. However, it may be that prophylactic colectomy can be temporarily postponed in AFAP when management by polypectomy is possible. Endoscopic surveillance of the rectum and anus should be continued after prophylactic colectomy. A baseline upper endoscopy is advisable by age 20, with follow-up examinations every two to three years unless symptoms occur. Annual thyroid palpation is suggested, and children at risk for FAP should have serum α-fetoprotein testing every 6 months until 6 years of age in the interest of early detection of hepatoblastoma.
When duodenal adenomas are discovered, they should be removed endoscopically if feasible. Often, however, the adenomas are too numerous to remove; in that case, annual surveillance with biopsy of grossly suspicious lesions is a prudent course. It is hoped that chemoprevention will help control duodenal adenomas, but results thus far have not been encouraging [reviewed by Hawk and colleagues (79)].
Desmoid tumors can be a difficult management problem. These locally aggressive soft-tissue tumors typically arise in the abdominal wall or bowel mesentery. Relentless recurrences are the rule (80,81). The difficulty of surgical cure, complete with the fact that surgery may initiate the pathogenesis of desmoids, has led to the recommendation that only symptomatic desmoid tumors should be surgically resected (82). Desmoid tumors may occur in excess in certain FAP families (81,83,84).
MYH (MUTYH) mutations and CRC
MUTYH is a DNA-base-excision-repair gene located on chromosome 1p. Mutations in this gene predispose to an autosomal recessively inherited colonic polyposis that shows an average of 55 adenomas in an affected individual (85) and carries a risk for CRC of approximately 60% (86). The disorder, termed MUTYH-associated polyposis (MAP) is rare, occurring in approximately 1 out of 10,000 in the general population and accounting for about 5% of persons with a FAP-type phenotype (85). It is estimated that 1.5% of individuals in the general population are heterozygous carriers of a MUTYH mutation (86).
Nielsen et al. (86) combined data from the literature as well as 40 Dutch MAP patients in order to construct a Markov model for developing a societal cost-utility analysis of genetic screening of MAP families, which involved testing the spouse when heterozygous and testing the progeny of that spouse. They recommended that counselees be offered the option of genetic testing of spouses and/or children. Biallelic MUTYH mutations are found in 10-25% of patients with “..between 10 and a few hundred adenomas and in 1% of patients with a colorectal carcinoma. Patients with more than 10 adenomas are currently being offered MUTYH mutation analysis. Siblings of a MAP patient have a 25% risk of having inherited biallelic mutations and are eligible for genetic testing. (86)” MAP patients have between 10 to about 100 adenomas at a mean age of 50 years; they are advised to have colonoscopic screening beginning at age 25 years.
Juvenile polyposis syndrome
Sporadic juvenile polyps are relatively common, with one autopsy study of patients under 21 years reporting a prevalence of 1% (87) and other studies estimating an occurrence in 2% of the pediatric population (88). Usually solitary, the polyps do not denote an increased risk for CRC (88,89).
Juvenile polyposis syndrome (JPS) is a rare autosomal dominantly inherited disorder characterized by multiple juvenile polyps in the gastrointestinal tract and an increased risk for gastrointestinal cancer. Mutations in SMADH4 and BMPR1A together account for approximately 50-60% of JPS cases. Various candidate genes have been investigated to account for the remainder of the disorder (88).
The clinical diagnosis of JPS requires histologic confirmation of a juvenile polyp, plus the presence of any of the following: more than five juvenile polyps in the colorectum, juvenile polyps elsewhere in the gastrointestinal tract, or a family history of juvenile polyposis. Even when there are multiple juvenile polyps and a family history of polyposis, the diagnosis of JPS must be made with care, because Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome have similar polyps but a very different spectrum of associated lesions (90).
Three variants of JPS have been described: a rare, usually fatal juvenile polyposis of infancy with diarrhea, protein-losing enteropathy, and alopecia; juvenile polyps of the colorectum; and generalized juvenile polyposis. However, the latter two are likely manifestations of the same disorder. Extraintestinal anomalies, while not common, have been reported, including hydrocephalus, thyroglossal duct cyst, tetralogy of Fallot, coarctation of the aorta, idiopathic hypertrophic subaortic stenosis, and malrotation of the gut.
Juvenile polyps may be found in the colorectum (98%), stomach (14%), duodenum (2%), and small bowel (7%) (91,92). Although the polyps are considered benign, JPS patients are at increased risk for CRC. The cumulative lifetime risk was estimated by Järvinen to be 50% (93), an estimate supported by a report from the University of Iowa describing a large JPS kindred in which 16 of 29 (55%) affected individuals developed gastrointestinal cancer (94). Eleven members of the Iowa kindred had colon cancer and six had gastric cancer.
Affected individuals need regular endoscopic surveillance. Scott-Conner and colleagues (92) recommend upper and lower gastrointestinal endoscopy beginning at age 15 and continuing every three years as long as no lesions are detected. If a family carries a SMADH4 or BMPR1A mutation, at-risk individuals can be tested to determine whether surveillance is needed. Small numbers of polyps can be managed by polypectomy, in which case endoscopy should be repeated yearly until the patient is free of polyps. If there are multiple polyps in the colon, subtotal colectomy is recommended if the rectum can be cleared endoscopically; otherwise, total colectomy is a consideration. For young children with large numbers of polyps, regular colonoscopy with removal of the largest polyps is an option as a temporary measure until puberty is reached (94). Multiple gastric polyps, particularly if dysplasia is present, should prompt consideration of gastrectomy.
Peutz-Jeghers syndrome
PJS is inherited in an autosomal dominant pattern and has been mapped to a locus on chromosome 19p13.3 (95,96). The gene responsible for the syndrome encodes a serine threonine kinase, STK11 (also known as LKB1) (95). Abnormal (i.e., inactive) forms of this kinase may lead to defective control of cellular growth and differentiation. Not all PJS families can be linked to 19p13.3, leading to speculation that there are other loci for the syndrome (97,98). The incidence of PJS is approximately 1 in 200,000 (99).
The diagnosis of PJS requires histologic confirmation of a hamartomatous, Peutz-Jeghers-type polyp. Because such polyps can be seen in individuals who do not have PJS, clinical diagnosis also requires at least two of the following: small bowel polyposis, family history of PJS; or pigmented macules of buccal mucosa, lips, fingers, and toes (100).
Peutz-Jeghers polyps have been found in the entire gastrointestinal tract. The small bowel is the site affected most often, but stomach and colon are involved as well; esophageal polyps are rare. The polyps are almost always multiple but tend to number in the dozens rather than the hundreds. Peutz-Jeghers polyps have also been described in respiratory mucosa and the urinary tract. One member of the Dutch family originally studied by Peutz suffered from severe nasal polyposis and eventually developed a nasal carcinoma (101).
PJS carries an increased risk for malignancy. Giardiello and colleagues (100) found a relative risk for cancer 18 times that of the general population in 31 PJS patients. Malignancies involved pancreas [4], breast [2], stomach [2], colon [2], lung [2], and endometrium [1]. Spigelman and colleagues (102) reported that 72 retrospectively studied PJS patients were 13 times more likely than the general population to develop a malignancy. The tumors involved colon, stomach, small intestine, ovary, fallopian tube, thyroid, and lung. Investigators from the Mayo Clinic (103) found a relative risk for cancer of 9.9 in 34 PJS patients, with cancers of the colon [7], breast [6], lung [3], and cervix [2] predominating. The Mayo Clinic group found a particularly high incidence of breast and gynecologic cancers, contributing to a relative cancer risk of 18.5 in women, compared to 6.2 in men.
The Dutch family originally described by Peutz has been updated, and the findings further demonstrate that PJS is a cancer-prone condition (101). Of 22 affected individuals, seven developed carcinoma (3 colon, 1 stomach, 1 gastrointestinal not otherwise specified, 1 breast, and 1 nasal cavity). All patients with malignancy were dead of their disease before the age of 50 years.
Nearly every female PJS patient will have ovarian involvement by sex cord tumor with annular tubules (SCTAT). The tumors are bilateral in at least two-thirds of cases (in contrast to SCTAT in the sporadic setting, which is almost always unilateral). An unusual form of cervical cancer, minimal deviation adenocarcinoma (adenoma malignum), is also characteristic of PJS. This rare tumor accounts for 1% to 3% of all cervical adenocarcinoma but, in one series, affected 4 of 27 women with PJS (104).
The surveillance protocol advocated by the St. Mark’s Polyposis Registry (105) includes yearly hemoglobin and yearly ultrasound of the pelvis in females and of the pancreas in all patients. Testicular ultrasound should also be done in males with feminizing features. Biannual upper and lower endoscopy with small-bowel X-ray are recommended. Regular mammography and cervical smear are critical surveillance measures. Tomlinson and Houlston (106) suggest that upper endoscopy, colonoscopy, and small-bowel X-ray begin in the second decade and that mammography begin at age 25.
The gastrointestinal polyps may be associated with bleeding, obstruction, or intussusception. Conservative removal (snare polypectomy) is favored over segmental resection of bowel to avoid development of a short bowel syndrome.
Differentiating among JPS, Cowden syndrome, Bannayan-Ruvalcaba-Riley syndrome, and PJS can be difficult (90).
Importance of family studies
A patient found to have CRC may eventually be diagnosed as having an underlying hereditary cancer syndrome. The individual may be found to have a germline mutation in one of the several MMR genes and thus to have LS, in the APC gene thus causing FAP, or in one of the genes associated with a more uncommon syndrome. The diagnosis may be strongly suspected on the basis of a known family history of colorectal and extracolonic cancers or, in the case of LS, may be made through the performance of MSI or IHC testing. Perhaps more important than the diagnosis of a hereditary CRC syndrome in an individual patient is that such a diagnosis makes it possible to provide predictive mutational testing for at-risk relatives. Relatives found to be negative for the familial mutation can be spared the anxiety, cost, and risk associated with unnecessary clinical surveillance. Those found to be positive for the mutation can receive the benefit of intensive surveillance and, perhaps, surgical prophylaxis intended to reduce risk of cancer-associated morbidity and mortality.
In reality, however, relatively few at-risk relatives of CRC patients have benefitted from predisposition testing. This issue extends to families with other hereditary cancer-prone syndromes such as the hereditary breast-ovarian cancer (HBOC) syndrome caused by BRCA germline mutations.
Attention is sometimes given to the proband’s first-degree relatives (siblings, parents, and progeny), and there is a modestly encouraging uptake of genetic testing seen among these family members. Rarely, however, is attention extended to more distant relatives who could benefit from genetic testing and counseling; it is not surprising that uptake of such procedures is more disappointing among these relatives. Such a difference is understandable, as most people are in more frequent contact with first-degree relatives and are highly motivated to seek their welfare. Communication with more distant relatives is less frequent and their health may not have the same perceived importance.
Watson et al. (107) studied Creighton’s LS and HBOC families to show how the results of genetic testing can clarify the cancer risk status of even family members who are not tested. Testing of 1,408 family members effected a change in known carrier risk status of 2,906 individuals. Sixty percent of those who had a change in status were not tested themselves; their risk status changed because of the test result of another relative. Carrier risk status changes from uncertainty to certainty (that is, from high risk to known carrier or noncarrier status) accounted for 89% of the changes, affecting cancer prevention recommendations.
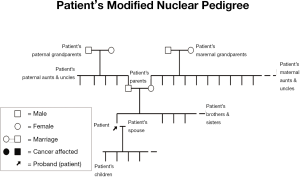
This current lack of detailed attention to study of the family, especially of relatives more distant than first-degree, may be depriving family members of cancer prevention, appropriate genetic counseling and, where indicated, DNA testing. It is clear that developing the family history (see Figure 3) and thereby implementing appropriate screening and DNA testing to determine mutation status of family members, followed by appropriate management implications, could provide highly-targeted cancer preventive management.
Acknowledgements
Disclosure: The authors declare no conflicts of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer, 2010. Available online: http://www-dep.iarc.fr/. Accessed: August 30, 2012.
- Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol 2008;26:5783-8. [PubMed]
- Peltomäki P. Lynch syndrome genes. Fam Cancer 2005;4:227-32. [PubMed]
- Bronner CE, Baker SM, Morrison PT, et al. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature 1994;368:258-61. [PubMed]
- Fishel R, Lescoe MK, Rao MR, et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 1993;75:1027-38. [PubMed]
- Leach FS, Nicolaides NC, Papadopoulos N, et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 1993;75:1215-25. [PubMed]
- Miyaki M, Konishi M, Tanaka K, et al. Germline mutation of MSH6 as the cause of hereditary nonpolyposis colorectal cancer. Nat Genet 1997;17:271-2. [PubMed]
- Nicolaides NC, Papadopoulos N, Liu B, et al. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature 1994;371:75-80. [PubMed]
- Vasen HF, Wijnen JT, Menko FH, et al. Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology 1996;110:1020-7. [PubMed]
- Rodríguez-Bigas MA, Vasen HF, Pekka-Mecklin J, et al. Rectal cancer risk in hereditary nonpolyposis colorectal cancer after abdominal colectomy. International Collaborative Group on HNPCC. Ann Surg 1997;225:202-7. [PubMed]
- Lynch HT, Lynch PM, Lanspa SJ, et al. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet 2009;76:1-18. [PubMed]
- Lynch HT, Lynch JF, Attard TA. Diagnosis and management of hereditary colorectal cancer syndromes: Lynch syndrome as a model. CMAJ 2009;181:273-80. [PubMed]
- Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003;348:919-32. [PubMed]
- Watson P, Lynch HT. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer 1993;71:677-85. [PubMed]
- Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet 1997;6:105-10. [PubMed]
- Hamilton SR, Liu B, Parsons RE, et al. The molecular basis of Turcot’s syndrome. N Engl J Med 1995;332:839-47. [PubMed]
- Lynch HT, Fusaro RM. Muir-Torre syndrome: heterogeneity, natural history, diagnosis, and management. Prob Gen Surg 1993;10:1-14.
- Risinger JI, Barrett JC, Watson P, et al. Molecular genetic evidence of the occurrence of breast cancer as an integral tumor in patients with the hereditary nonpolyposis colorectal carcinoma syndrome. Cancer 1996;77:1836-43. [PubMed]
- Lynch HT, de la Chapelle A. Genetic susceptibility to non-polyposis colorectal cancer. J Med Genet 1999;36:801-18. [PubMed]
- Nicolaides NC, Littman SJ, Modrich P, et al. A naturally occurring hPMS2 mutation can confer a dominant negative mutator phenotype. Mol Cell Biol 1998;18:1635-41. [PubMed]
- Wijnen J, de Leeuw W, Vasen H, et al. Familial endometrial cancer in female carriers of MSH6 germline mutations. Nat Genet 1999;23:142-4. [PubMed]
- Vasen HF, Stormorken A, Menko FH, et al. MSH2 mutation carriers are at higher risk of cancer than MLH1 mutation carriers: a study of hereditary nonpolyposis colorectal cancer families. J Clin Oncol 2001;19:4074-80. [PubMed]
- Hendriks YM, Wagner A, Morreau H, et al. Cancer risk in hereditary nonpolyposis colorectal cancer due to MSH6 mutations: impact on counseling and surveillance. Gastroenterology 2004;127:17-25. [PubMed]
- Bandipalliam P. Syndrome of early onset colon cancers, hematologic malignancies & features of neurofibromatosis in HNPCC families with homozygous mismatch repair gene mutations. Fam Cancer 2005;4:323-33. [PubMed]
- de Vos M, Hayward B, Bonthron DT, et al. Phenotype associated with recessively inherited mutations in DNA mismatch repair (MMR) genes. Biochem Soc Trans 2005;33:718-20. [PubMed]
- Scott RH, Homfray T, Huxter NL, et al. Familial T-cell non-Hodgkin lymphoma caused by biallelic MSH2 mutations. J Med Genet 2007;44:e83. [PubMed]
- Pineda M, Castellsagué E, Musulén E, et al. Non-Hodgkin lymphoma related to hereditary nonpolyposis colorectal cancer in a patient with a novel heterozygous complex deletion in the MSH2 gene. Genes Chromosomes Cancer 2008;47:326-32. [PubMed]
- Menko FH, Kaspers GL, Meijer GA, et al. A homozygous MSH6 mutation in a child with café-au-lait spots, oligodendroglioma and rectal cancer. Fam Cancer 2004;3:123-7. [PubMed]
- Wang Q, Lasset C, Desseigne F, et al. Neurofibromatosis and early onset of cancers in hMLH1-deficient children. Cancer Res 1999;59:294-7. [PubMed]
- Ricciardone MD, Ozçelik T, Cevher B, et al. Human MLH1 deficiency predisposes to hematological malignancy and neurofibromatosis type 1. Cancer Res 1999;59:290-3. [PubMed]
- De Rosa M, Fasano C, Panariello L, et al. Evidence for a recessive inheritance of Turcot's syndrome caused by compound heterozygous mutations within the PMS2 gene. Oncogene 2000;19:1719-23. [PubMed]
- Vilkki S, Tsao JL, Loukola A, et al. Extensive somatic microsatellite mutations in normal human tissue. Cancer Res 2001;61:4541-4. [PubMed]
- Trimbath JD, Petersen GM, Erdman SH, et al. Café-au-lait spots and early onset colorectal neoplasia: a variant of HNPCC? Fam Cancer 2001;1:101-5. [PubMed]
- Whiteside D, McLeod R, Graham G, et al. A homozygous germ-line mutation in the human MSH2 gene predisposes to hematological malignancy and multiple café-au-lait spots. Cancer Res 2002;62:359-62. [PubMed]
- Bougeard G, Charbonnier F, Moerman A, et al. Early onset brain tumor and lymphoma in MSH2-deficient children. Am J Hum Genet 2003;72:213-6. [PubMed]
- Gallinger S, Aronson M, Shayan K, et al. Gastrointestinal cancers and neurofibromatosis type 1 features in children with a germline homozygous MLH1 mutation. Gastroenterology 2004;126:576-85. [PubMed]
- Broaddus RR, Lynch PM, Lu KH, et al. Unusual tumors associated with the hereditary nonpolyposis colorectal cancer syndrome. Mod Pathol 2004;17:981-9. [PubMed]
- Berends MJ, Cats A, Hollema H, et al. Adrenocortical adenocarcinoma in an MSH2 carrier: coincidence or causal relation? Hum Pathol 2000;31:1522-7. [PubMed]
- Lynch HT, Shaw MW, Magnuson CW, et al. Hereditary factors in cancer. Study of two large midwestern kindreds. Arch Intern Med 1966;117:206-12. [PubMed]
- Sijmons R, Hofstra R, Hollema H, et al. Inclusion of malignant fibrous histiocytoma in the tumour spectrum associated with hereditary non-polyposis colorectal cancer. Genes Chromosomes Cancer 2000;29:353-5. [PubMed]
- Boyd J, Rhei E, Federici MG, et al. Male breast cancer in the hereditary nonpolyposis colorectal cancer syndrome. Breast Cancer Res Treat 1999;53:87-91. [PubMed]
- Kovacs ME, Papp J, Szentirmay Z, et al. Deletions removing the last exon of TACSTD1 constitute a distinct class of mutations predisposing to Lynch syndrome. Hum Mutat 2009;30:197-203. [PubMed]
- Kobelka CE. Silencing is not-so golden: a new model for inheritance of Lynch syndrome. Clin Genet 2009;75:522-3.
- Ligtenberg MJ, Kuiper RP, Chan TL, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3' exons of TACSTD1. Nat Genet 2009;41:112-7. [PubMed]
- Kuiper RP, Vissers LE, Venkatachalam R, et al. Recurrence and variability of germline EPCAM deletions in Lynch syndrome. Hum Mutat 2011;32:407-14. [PubMed]
- Lynch HT, Riegert-Johnson DL, Snyder C, et al. Lynch syndrome-associated extracolonic tumors are rare in two extended families with the same EPCAM deletion. Am J Gastroenterol 2011;106:1829-36. [PubMed]
- Lindor NM, Rabe K, Petersen GM, et al. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: familial colorectal cancer type X. JAMA 2005;293:1979-85. [PubMed]
- Llor X, Pons E, Xicola RM, et al. Differential features of colorectal cancers fulfilling Amsterdam criteria without involvement of the mutator pathway. Clin Cancer Res 2005;11:7304-10. [PubMed]
- Valle L, Perea J, Carbonell P, et al. Clinicopathologic and pedigree differences in amsterdam I-positive hereditary nonpolyposis colorectal cancer families according to tumor microsatellite instability status. J Clin Oncol 2007;25:781-6. [PubMed]
- Cunningham JM, Christensen ER, Tester DJ, et al. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res 1998;58:3455-60. [PubMed]
- Marcus VA, Madlensky L, Gryfe R, et al. Immunohistochemistry for hMLH1 and hMSH2: a practical test for DNA mismatch repair-deficient tumors. Am J Surg Pathol 1999;23:1248-55. [PubMed]
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med 2009;11:35-41. [PubMed]
- Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis. Ann Intern Med 2011;155:69-79. [PubMed]
- Burt RW. Who should have genetic testing for the Lynch syndrome? Ann Intern Med 2011;155:127-8. [PubMed]
- Teutsch SM, Bradley LA, Palomaki GE, et al. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Initiative: methods of the EGAPP Working Group. Genet Med 2009;11:3-14. [PubMed]
- Mvundura M, Grosse SD, Hampel H, et al. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med 2010;12:93-104. [PubMed]
- Vasen HF, Möslein G, Alonso A, et al. Recommendations to improve identification of hereditary and familial colorectal cancer in Europe. Fam Cancer 2010;9:109-15. [PubMed]
- Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 2000;118:829-34. [PubMed]
- Dove-Edwin I, Sasieni P, Adams J, et al. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study. BMJ 2005;331:1047. [PubMed]
- Jass JR, Stewart SM. Evolution of hereditary non-polyposis colorectal cancer. Gut 1992;33:783-6. [PubMed]
- Vasen HF, Nagengast FM, Khan PM. Interval cancers in hereditary non-polyposis colorectal cancer (Lynch syndrome). Lancet 1995;345:1183-4. [PubMed]
- Church J. Hereditary colon cancers can be tiny: a cautionary case report of the results of colonoscopic surveillance. Am J Gastroenterol 1998;93:2289-90. [PubMed]
- Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112:24-8. [PubMed]
- Lynch HT. Is there a role for prophylactic subtotal colectomy among hereditary nonpolyposis colorectal cancer germline mutation carriers? Dis Colon Rectum 1996;39:109-10. [PubMed]
- Church JM. Prophylactic colectomy in patients with hereditary nonpolyposis colorectal cancer. Ann Med 1996;28:479-82. [PubMed]
- Syngal S, Weeks JC, Schrag D, et al. Benefits of colonoscopic surveillance and prophylactic colectomy in patients with hereditary nonpolyposis colorectal cancer mutations. Ann Intern Med 1998;129:787-96. [PubMed]
- Schmeler KM, Lynch HT, Chen LM, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med 2006;354:261-9. [PubMed]
- Powell SM, Petersen GM, Krush AJ, et al. Molecular diagnosis of familial adenomatous polyposis. N Engl J Med 1993;329:1982-7. [PubMed]
- Bodmer WF, Bailey CJ, Bodmer J, et al. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature 1987;328:614-6. [PubMed]
- Leppert M, Dobbs M, Scambler P, et al. The gene for familial polyposis coli maps to the long arm of chromosome 5. Science 1987;238:1411-3. [PubMed]
- Kinzler KW, Nilbert MC, Su LK, et al. Identification of FAP locus genes from chromosome 5q21. Science 1991;253:661-5. [PubMed]
- Nishisho I, Nakamura Y, Miyoshi Y, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 1991;253:665-9. [PubMed]
- Powell SM, Zilz N, Beazer-Barclay Y, et al. APC mutations occur early during colorectal tumorigenesis. Nature 1992;359:235-7. [PubMed]
- Wallace MH, Phillips RK. Upper gastrointestinal disease in patients with familial adenomatous polyposis. Br J Surg 1998;85:742-50. [PubMed]
- Spirio L, Otterud B, Stauffer D, et al. Linkage of a variant or attenuated form of adenomatous polyposis coli to the adenomatous polyposis coli (APC) locus. Am J Hum Genet 1992;51:92-100. [PubMed]
- Spirio L, Olschwang S, Groden J, et al. Alleles of the APC gene: an attenuated form of familial polyposis. Cell 1993;75:951-7. [PubMed]
- Lynch HT, Smyrk T, McGinn T, et al. Attenuated familial adenomatous polyposis (AFAP). A phenotypically and genotypically distinctive variant of FAP. Cancer 1995;76:2427-33. [PubMed]
- Hawk E, Lubet R, Limburg P. Chemoprevention in hereditary colorectal cancer syndromes. Cancer 1999;86:2551-63. [PubMed]
- Lynch HT, Fitzgibbons R Jr, Chong S, et al. Use of doxorubicin and dacarbazine for the management of unresectable intra-abdominal desmoid tumors in Gardner,s syndrome. Dis Colon Rectum 1994;37:260-7. [PubMed]
- Lynch HT, Fitzgibbons R Jr. Surgery, desmoid tumors, and familial adenomatous polyposis: case report and literature review. Am J Gastroenterol 1996;91:2598-601. [PubMed]
- Rodriguez-Bigas MA, Mahoney MC, Karakousis CP, et al. Desmoid tumors in patients with familial adenomatous polyposis. Cancer 1994;74:1270-4. [PubMed]
- Lynch HT. Desmoid tumors: genotype-phenotype differences in familial adenomatous polyposis--a nosological dilemma. Am J Hum Genet 1996;59:1184-5. [PubMed]
- Lynch HT, Thorson AG, McComb RD, et al. Familial adenomatous polyposis and extracolonic cancer. Dig Dis Sci 2001;46:2325-32. [PubMed]
- Sieber OM, Lipton L, Crabtree M, et al. Multiple colorectal adenomas, classic adenomatous polyposis, and germ-line mutations in MYH. N Engl J Med 2003;348:791-9. [PubMed]
- Nielsen M, Hes FJ, Vasen HF, et al. Cost-utility analysis of genetic screening in families of patients with germline MUTYH mutations. BMC Med Genet 2007;8:42. [PubMed]
- Helwig EB. Adenomas of the large intestine in children. Am J Dis Child 1946;72:289-95. [PubMed]
- Brosens LA, Langeveld D, van Hattem WA, et al. Juvenile polyposis syndrome. World J Gastroenterol 2011;17:4839-44. [PubMed]
- Nugent KP, Talbot IC, Hodgson SV, et al. Solitary juvenile polyps: not a marker for subsequent malignancy. Gastroenterology 1993;105:698-700. [PubMed]
- Eng C, Ji H. Molecular classification of the inherited hamartoma polyposis syndromes: clearing the muddied waters. Am J Hum Genet 1998;62:1020-2. [PubMed]
- Desai DC, Neale KF, Talbot IC, et al. Juvenile polyposis. Br J Surg 1995;82:14-7. [PubMed]
- Scott-Conner CE, Hausmann M, Hall TJ, et al. Familial juvenile polyposis: patterns of recurrence and implications for surgical management. J Am Coll Surg 1995;181:407-13. [PubMed]
- Järvinen HJ. Juvenile gastrointestinal polyposis. Prob Clin Surg 1993;10:749-57.
- Howe JR, Mitros FA, Summers RW. The risk of gastrointestinal carcinoma in familial juvenile polyposis. Ann Surg Oncol 1998;5:751-6. [PubMed]
- Jenne DE, Reimann H, Nezu J, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet 1998;18:38-43. [PubMed]
- Hemminki A, Tomlinson I, Markie D, et al. Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis. Nat Genet 1997;15:87-90. [PubMed]
- Olschwang S, Markie D, Seal S, et al. Peutz-Jeghers disease: most, but not all, families are compatible with linkage to 19p13.3. J Med Genet 1998;35:42-4. [PubMed]
- Mehenni H, Blouin JL, Radhakrishna U, et al. Peutz-Jeghers syndrome: confirmation of linkage to chromosome 19p13.3 and identification of a potential second locus, on 19q13.4. Am J Hum Genet 1997;61:1327-34. [PubMed]
- Schreibman IR, Baker M, Amos C, et al. The hamartomatous polyposis syndromes: a clinical and molecular review. Am J Gastroenterol 2005;100:476-90. [PubMed]
- Giardiello FM, Welsh SB, Hamilton SR, et al. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med 1987;316:1511-4. [PubMed]
- Westerman AM, Entius MM, de Baar E, et al. Peutz-Jeghers syndrome: 78-year follow-up of the original family. Lancet 1999;353:1211-5. [PubMed]
- Spigelman AD, Murday V, Phillips RK. Cancer and the Peutz-Jeghers syndrome. Gut 1989;30:1588-90. [PubMed]
- Boardman LA, Thibodeau SN, Schaid DJ, et al. Increased risk for cancer in patients with the Peutz-Jeghers syndrome. Ann Intern Med 1998;128:896-9. [PubMed]
- Young RH, Welch WR, Dickersin GR, et al. Ovarian sex cord tumor with annular tubules: review of 74 cases including 27 with Peutz-Jeghers syndrome and four with adenoma malignum of the cervix. Cancer 1982;50:1384-402. [PubMed]
- Spigelman AD, Arese P, Phillips RK. Polyposis: the Peutz-Jeghers syndrome. Br J Surg 1995;82:1311-4. [PubMed]
- Tomlinson IP, Houlston RS. Peutz-Jeghers syndrome. J Med Genet 1997;34:1007-11. [PubMed]
- Watson P, Narod SA, Fodde R, et al. Carrier risk status changes resulting from mutation testing in hereditary non-polyposis colorectal cancer and hereditary breast-ovarian cancer. J Med Genet 2003;40:591-6. [PubMed]


