Stage II colon cancer
Introduction
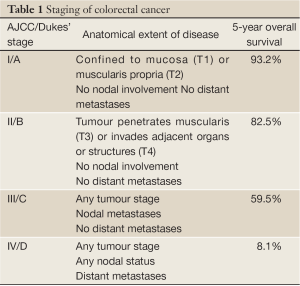
Colorectal cancer (CRC) is the third commonest cancer in the Western world, with an estimated 142,570 cases diagnosed in the US in 2010 (SEER database: http://seer.cancer.gov). Worldwide approximately 1.23 million new cases are diagnosed each year and 608,000 deaths from CRC occurred in 2008 (1). Overall, one quarter of incident cases are stage II, meaning that the tumour has breached the muscularis (T3) and may invade adjacent organs (T4), but has not spread to draining lymph nodes or distant sites (Table 1). However this proportion varies with tumour site, as almost a third of colonic cancers are stage II compared with just over one fifth of rectal cancers (SEER database: http://seer.cancer.gov). Stage II CRC is a heterogeneous disease both clinically and biologically. For instance, the risk of relapse following resection of a microsatellite unstable T3 lesion may less than 10%, while a patient who undergoes surgery for a mismatch repair proficient T4 tumour may have a risk of disease recurrence greater than 50%. The overrepresentation of microsatellite instability in stage II tumours compared to CRC overall also illustrates the variability in CRC biology at differing disease stages. In view of this heterogeneity it is unsurprising that the benefits of adjuvant chemotherapy for stage II CRC vary widely depending on classical histopathological and molecular tumour features.
Full Table
In this Review, we present an updated summary on the diagnosis and staging, pathological analysis, and therapeutic management of stage II CRC. We limit our discussion to colonic tumours (approximately two thirds of the total), as the management of rectal cancers differs substantially and is reviewed elsewhere in this issue. In addition to providing a précis of stage II colonic cancers we focus particularly on the evolving role of biomarkers in predicting the risk of relapse and guiding decisions on adjuvant therapy.
Diagnosis and staging of CRC
In the absence of screening, CRC is usually diagnosed following symptoms from the primary tumour or metastases. Population analyses have shown that approximately one quarter of all colorectal cancers in an unscreened population are stage II. Interestingly, though it might be hypothesized that the introduction of screening would result in an increase in the proportion of stage II tumours this was not the case in several screening studies (1-3), in which stage migration following implementation of fecal occult blood testing was mainly manifest as an increase in stage I and a reduction in stage IV disease. Consequently, the widespread adoption of screening may not result in a substantial alteration in the frequency of stage II CRC.
While surgical resection of most stage II colonic tumours by open or laparoscopic surgery is straightforward, the management of T4 cancers invading adjacent structures is more challenging. The role of imaging in predicting resectability has evolved substantially in recent years, and in our unit consideration is given to the use of preoperative chemotherapy with aim of facilitating surgery in patients with advanced T4 lesions.
Following resection, accurate pathological assessment is essential to confirm diagnosis of stage II disease, with examination of a minimum 12 lymph nodes recommended by consensus guidelines (4), although evidence suggests that prognosis of stage II disease improves according to the number of nodes analysed - suggesting that a proportion of patients with occult nodal metastases are under-staged by suboptimal pathological evaluation (5-8). The extent of tumour invasion is also essential in informing further management, as is the presence or absence of microsatellite instability (MSI) (discussed below). Other pathological features commonly suggested to be of prognostic import, but in some cases unvalidated are tumour vascular invasion and grade. Though often taken for granted in everyday practice, the pathologist’s role in determination of these factors is of pivotal importance in informing subsequent patient management.
Biology of stage II colon cancer
Although there are commonalities with other stages of CRC, there are also notable differences between stage II colon cancer and other disease stages. The most well recognized of these is the high frequency of MSI in stage II colon cancer, present in 15% of cases overall, and around 25% of right sided tumours, in comparison with a frequency of 14 in stage III colon cancer and 4% in metastatic disease (9). Mismatch repair proteins are required for surveillance of the newly synthesized DNA strand following replication, where they serve to recognize mispaired bases, small insertions and deletions incorporated by DNA polymerases (10). Germline mutation of the mismatch repair genes MLH1, MSH2, MSH6 or PMS2 causes Lynch syndrome (also known as hereditary non-polyposis colorectal cancer - HNPCC), associated with early onset colonic and endometrial cancer, in addition to tumours of the ovary, stomach, small bowel, pancreas and other sites (11,12). Defective mismatch repair function in sporadic colonic cancer is commonly due to mutation of MSH6, MSH2 or epigenetic silencing of MLH1 by promoter methylation (12). In both hereditary and sporadic tumours, aberrant mismatch repair function leads to failure to repair defects caused by slippage of DNA polymerases at microsatellites - short tandem DNA repeats - and point mutations, resulting in a characteristic molecular phenotype of microsatellite instability (MSI) and mutation of the tumour suppressors TGFβR2, IGF2R, BAX, and PTEN, and the oncogene BRAF (12-15). MSI-high tumours are commonly proximal to the splenic flexure, poorly differentiated and demonstrate a prominent lymphocytic infiltrate (12). Confirmation of tumour microsatellite instability can be performed either using PCR - by the demonstration of instability of at least 2 of 5 microsatellite markers examined - or by immunohistochemistry (IHC) for the mismatch repair proteins, as absent staining demonstrates excellent concordance with MSI-high status (16,17). Testing for MSI in stage II colonic cancer, and particularly in T3 tumours is advised, as it has important prognostic and therapeutic implications, as discussed below.
Adjuvant chemotherapy for stage II colonic cancer
Although the benefits of adjuvant 5-fluorouracil (FU) chemotherapy following resection of stage III disease have been well recognized for over two decades, the role of postoperative chemotherapy for stage II disease remained unclear until the publication of the QUASAR trial. This study randomized 3,239 patients following resection of CRC, 90% of whom had stage II disease, to adjuvant chemotherapy with FU and folinic acid (n=1,622) or to observation (n=1,617). After a median follow-up of 5.5 years, the recurrence rate in the chemotherapy arm was 20% lower than in the observation arm, translating to an absolute reduction in risk of relapse of 3.6% (P=0.04) (18). This unequivocal demonstration of the benefit of adjuvant chemotherapy for stage II colonic cancer is supported by other analyses (19-22) and means that an informed discussion of the risks and benefits of treatment is essential with fit patients following surgery. The MOSAIC study demonstrated that the addition of oxaliplatin to FU improves recurrence-free survival following surgery for stage III disease albeit at the expense of greater toxicity (23). However, subsequent data from this trial indicate that although this translated to a survival benefit at 6 years from combination therapy for stage III disease, no advantage was evident for stage II cancers (24). Consequently, oxaliplatin cannot be routinely recommended for use as adjuvant therapy in stage II colon cancer.
Biomarkers in stage II CRC
In view of the modest overall benefit from adjuvant chemotherapy in stage II colon cancer attempts have been made to restrict its use to patients with high-risk disease, on the premise that such patients are most likely to gain from therapy. The criteria used to identify ‘bad prognostic factors’ - T4 primary, high grade, lymphovascular invasion etc - have generally been identified by retrospective subgroup analysis, and although the prognostic significance of T4 primary is well recognized (25), most other factors have not been validated prospectively. Indeed, when reflecting on the quality of the underlying data, it is puzzling that some such features have gained traction in clinical practice and been included in treatment guidelines for stage II disease. However, recent high-quality data from the molecular analysis of large prospective clinical trials has clearly demonstrated the prognostic significance of tumour microsatellite instability, and suggested that the analysis of tumour gene expression profiles may aid in treatment decisions in some cases of colon cancer.
Prognostic significance of microsatellite instability (MSI)
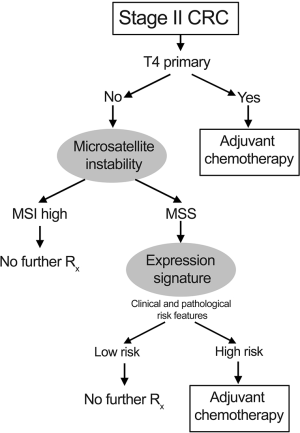
Although the prognostic significance of MSI was previously unclear, data from several large randomized clinical trials (RCTs) (9,26-30), and a meta-analysis (31) have conclusively proven that the presence of tumour MSI is associated with favourable outcome. The meta analysis of 7,642 patients, 1,277 of whom had MSI tumours showed a hazard ratio for death of 0.65 (95% CI, 0.59 to 0.71) for patients with MSI tumours compared to those with microsatellite stable (MSS) disease (31). Even disregarding the suggestion that MSI may predict lack of benefit from adjuvant chemotherapy (31), patients with T3 primary and tumour MSI have sufficiently low risk of recurrence to mean that any benefit from post-operative chemotherapy is minimal, and these patients can therefore be spared treatment. Interestingly, the combination of T4 primary and MSI is uncommon - around 2% of cases of stage II colon cancer - and appears to have similar prognosis to that of T3 primary, MSS disease, although there is a large degree of uncertainty in this estimate. Consequently, consideration of adjuvant chemotherapy should be given to patients this group. The mechanisms underlying the favourable outcome of MSI-high cancers is presently unclear, but may be due to and anti-tumour immune response (32) or decreased viability associated with hypermutation in tumours (33). Data regarding the utility of adjuvant chemotherapy for the majority of patients (85%) with MSS tumours, are insufficiently strong to alter the estimated benefit from the QUASAR study (18). A proposed treatment algorithm, accounting for tumour stage and mismatch repair status is shown in Figure 1.
Prognostic gene signatures
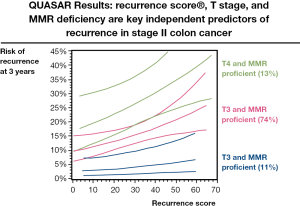
As an attempt to improve on the utility of conventional clinicopathological features for prognostication in stage II colon cancer, a transatlantic collaboration between QUASAR and NSABP Trials Groups, Cleveland Clinic and Genomic Health, was formed. This collaborative effort sought to examine whether tumour RNA expression levels might serve to improve on conventional parameters for the classification of relapse risk. The developmental study comprised 1,851 patients recruited to NSABP clinical trials C-01/C-02/C-04/C-06 and a cohort of untreated patients from the Cleveland clinic (34). RNA was extracted from formalin-fixed paraffin embedded (FFPE) tumour blocks, and gene expression quantified by RT-PCR. Multivariate analysis of the correlation of expression of 761 candidate genes on recurrence-free survival (RFS), disease-free survival (DFS), and overall survival (OS) adjusted for stage, grade, number of lymph nodes examined and MSI status, yielded 18 informative genes (7 prognostic genes, 6 genes predictive of FU benefit and 5 internal reference genes for normalisation), which were used to generate separate prognostic recurrence score and predictive treatment score signatures. The utility of these gene expression scores was then examined in 1,436 patients with median follow-up of 6.6 years from the QUASAR study (35). In univariate analysis, the recurrence score predicted recurrence risk (hazard ratio/25 units =1.58; 95% CI, 1.15 to 2.15; P=0.004), DFS (P=0.01) and OS (P=0.04). Recurrence risk increased with increasing recurrence score, with 3- year recurrences of 12, 18 and 22% in the predefined low, intermediate and high recurrence risk groups (Figure 2). In multivariate analyses, the recurrence score retained prognostic significance (P=0.008) following adjustment for primary tumour stage, number of lymph nodes examined, MSI status, tumour grade, and tumour lymphovascular invasion. However, the treatment score failed to predict chemotherapy benefit (P=0.19) (35). Thus, the continuous recurrence score is able to enhance the assessment of recurrence risk and may be of particular use for the majority (76%) of cases of stage II colon cancer with T3 MSS tumours, as shown in Figure 2. In this group, the recurrence score can be used to segregate those into very low risk of relapse for whom the absolute benefits of chemotherapy are too small to recommend its use, from those at greater risk, for whom a 25-30% risk of recurrence is associated with a greater absolute benefit from adjuvant treatment - perhaps 5-6 percentage points. In the group at intermediate risk, a more informed discussion between the patient and clinician on the likely benefits of adjuvant chemotherapy than is currently possible may be undertaken. The recurrence score has the advantage of using conventional pathologic material, in contrast to alternatives that require frozen tissue - not routinely collected in everyday clinical practice. It is hoped that in the future, an improved predictive score for chemotherapy benefit will provide additional information that can be used to guide treatment decisions in patients with stage II colon cancer.
Conclusions
Approximately one quarter of patients with colorectal cancers have stage II disease, and within this group there is substantial variation in clinicopathological features, molecular biology and outcome between cases. The prognosis varies from the excellent outcome associated with MSI T3 primary to a recurrence risk of >50% for MSS T4 primary presenting with bowel obstruction. Consequently, as we have sought to highlight in this Review, a one-size-fits-all approach cannot be recommended, and treatment decisions must be individualized, informed by tumour stage and MSI status at the very least. In a proportion of cases, the recurrence score may provide further information on the risk of relapse than conventional clinicopathological features alone, and help in decision-making. Ongoing studies should clarify the role of additional molecular markers in assessment of prognosis and likelihood of chemotherapy benefit.
Acknowledgements
Disclosure: Dr Rachel Midgley is receiving research funding from Genomic Health with respect to a project looking for a potential gene expression signature as a marker of therapeutic efficacy. She gratefully acknowledges support from the DH and HEFCE (UK) in the form of a personal fellowship and from the Oxford Biomedical Research Council (BMRC). Dr Church acknowledges support from the Oxford BMRC. Professor David Kerr has previously received research funding from Genomic Health with respect to a project validating a gene expression signature for colorectal cancer prognosis.
References
- Gill MD, Bramble MG, Rees CJ, et al. Comparison of screen-detected and interval colorectal cancers in the Bowel Cancer Screening Programme. Br J Cancer 2012;107:417-21. [PubMed]
- Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348:1472-7. [PubMed]
- Faivre J, Dancourt V, Lejeune C, et al. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology 2004;126:1674-80. [PubMed]
- Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 2001;93:583-96. [PubMed]
- Choi HK, Law WL, Poon JT. The optimal number of lymph nodes examined in stage II colorectal cancer and its impact of on outcomes. BMC Cancer 2010;10:267. [PubMed]
- Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol 2001;19:157-63. [PubMed]
- Swanson RS, Compton CC, Stewart AK, et al. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol 2003;10:65-71. [PubMed]
- Tsai HL, Lu CY, Hsieh JS, et al. The prognostic significance of total lymph node harvest in patients with T2-4N0M0 colorectal cancer. J Gastrointest Surg 2007;11:660-5. [PubMed]
- Bertagnolli MM, Redston M, Compton CC, et al. Microsatellite instability and loss of heterozygosity at chromosomal location 18q: prospective evaluation of biomarkers for stages II and III colon cancer--a study of CALGB 9581 and 89803. J Clin Oncol 2011;29:3153-62. [PubMed]
- Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem 2005;74:681-710. [PubMed]
- Lynch HT, Boland CR, Gong G, et al. Phenotypic and genotypic heterogeneity in the Lynch syndrome: diagnostic, surveillance and management implications. Eur J Hum Genet 2006;14:390-402. [PubMed]
- Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010;138:2073-2087.e3.
- Ionov Y, Peinado MA, Malkhosyan S, et al. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993;363:558-61. [PubMed]
- Rampino N, Yamamoto H, Ionov Y, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 1997;275:967-9. [PubMed]
- Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993;260:816-9. [PubMed]
- Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248-57. [PubMed]
- Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol 2002;20:1043-8. [PubMed]
- Quasar Collaborative Group, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020-9. [PubMed]
- Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol 1999;17:1356-63. [PubMed]
- Figueredo A, Charette ML, Maroun J, et al. Adjuvant therapy for stage II colon cancer: a systematic review from the Cancer Care Ontario Program in evidence-based care’s gastrointestinal cancer disease site group. J Clin Oncol 2004;22:3395-407. [PubMed]
- Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22:1797-806. [PubMed]
- Mamounas E, Wieand S, Wolmark N, et al. Comparative efficacy of adjuvant chemotherapy in patients with Dukes’ B versus Dukes’ C colon cancer: results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C-01, C-02, C-03, and C-04). J Clin Oncol 1999;17:1349-55. [PubMed]
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343-51. [PubMed]
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16. [PubMed]
- O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 2004;96:1420-5.
- Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol 2010;28:466-74. [PubMed]
- Roth AD, Delorenzi M, Tejpar S, et al. Integrated analysis of molecular and clinical prognostic factors in stage II/III colon cancer. J Natl Cancer Inst 2012;104:1635-46. [PubMed]
- Halling KC, French AJ, McDonnell SK, et al. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst 1999;91:1295-303. [PubMed]
- Sinicrope FA, Rego RL, Halling KC, et al. Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology 2006;131:729-37. [PubMed]
- Watanabe T, Wu TT, Catalano PJ, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med 2001;344:1196-206. [PubMed]
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609-18. [PubMed]
- Dolcetti R, Viel A, Doglioni C, et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol 1999;154:1805-13. [PubMed]
- Sankila R, Aaltonen LA, Järvinen HJ, et al. Better survival rates in patients with MLH1-associated hereditary colorectal cancer. Gastroenterology 1996;110:682-7. [PubMed]
- O’Connell MJ, Lavery I, Yothers G, et al. Relationship between tumor gene expression and recurrence in four independent studies of patients with stage II/III colon cancer treated with surgery alone or surgery plus adjuvant fluorouracil plus leucovorin. J Clin Oncol 2010;28:3937-44. [PubMed]
- Gray RG, Quirke P, Handley K, et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol 2011;29:4611-9. [PubMed]


