Trastuzumab emtansine in HER2-positive metastatic breast cancer: what is the best sequence?
Human epidermal receptor growth factor 2 (HER2, ERBB2) gene amplification occurs in about 15–20% of invasive breast cancers and is associated with biologically aggressive disease and poor overall survival in the absence of systemic therapy (1). The synthesis of trastuzumab, a humanized monoclonal antibody targeting the extracellular domain of HER2, was a major leap forward in the treatment of this disease. In fact, it not only yielded, in combination with chemotherapy, a significant improvement in progression-free and overall survivals (2), but also allowed the evolution of the other HER2-targeted therapies, which currently represent the mainstay of treatment for locally advanced and metastatic breast cancer (mBC) with an HER2 amplification. On this basis, the therapeutic approach of HER2-positive mBC has been totally reconfigured following a trilogy of randomized clinical trials published between 2012 and 2014 (3-5). The results of these trials led to identify in the metastatic setting a sequence of treatment that is now commonly agreed (6). First, standard first line therapy for HER2-positive mBC is based on the combination of chemotherapy (namely a taxane) with a dual anti-HER2 blockade with trastuzumab and pertuzumab, a humanized monoclonal antibody targeting another HER2 epitope than trastuzumab (3). Second, second line of treatment is based on trastuzumab emtansine, an antibody-drug conjugate composed of the humanized monoclonal antibody trastuzumab stably linked to the cytotoxic microtubule inhibitor DM1 (4-6). Last, continuing HER2 blockade with trastuzumab in combination with several chemotherapeutic agents is preferable for subsequent lines of therapy, and the combinations of lapatinib [a dual kinase inhibitor that targets the intracellular domain of HER2 and epidermal growth factor receptor (EGFR)] with trastuzumab or capecitabine are reasonable treatment options for some patients (6).
However, as often happens when scientific evidence rapidly increases within a short period of time, the current place of a specific treatment within this therapeutic sequence does not always reflect the original design of the clinical trial that led to its validation. On the basis of the above-mentioned therapeutic sequence for HER2-positive mBC, trastuzumab emtansine currently represents the standard of care in second-line treatment. In fact, it is approved in many countries worldwide for the treatment of HER2 amplified mBC in patients who previously received trastuzumab and a taxane (separately or in combination), and who received previous therapy for mBC or developed disease recurrence within 6 months of completing adjuvant therapy. This approval is based on two phase 3 randomized trials (EMILIA and TH3RESA) that compared trastuzumab emtansine with the combination of lapatinib and capecitabine (EMILIA) or with a treatment of physician’s choice (TH3RESA) in patients with trastuzumab-resistant mBC (4,5). However, only 61% of patients in EMILIA received trastuzumab emtansine directly at first disease progression after previous treatment containing trastuzumab and a taxane (including those who had developed disease recurrence within 6 months of completing adjuvant therapy), while the remaining patients, as well as all the patients included in TH3RESA, were previously heavily pretreated and received trastuzumab emtansine at progression after at least two lines of treatment (a median of four previous treatment regimens for mBC, excluding hormonal monotherapy, in TH3RESA), none of which contained pertuzumab (a treatment not yet validated at the time of enrollment in both trials).
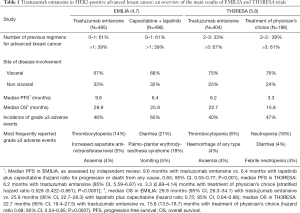
The final overall survival analyses of EMILIA (7) and TH3RESA (8) were recently published in The Lancet Oncology (Table 1). The descriptive analysis of overall survival in EMILIA showed that trastuzumab emtansine improved overall survival compared to the combination of capecitabine and lapatinib [29.9 versus 25.9 months; hazard ratio 0.75 (95% CI, 0.64–0.88)], although a substantial proportion of patients (27%) crossed over from the control arm to the trastuzumab emtansine arm after the second interim overall survival analysis. These results confirm that trastuzumab emtansine is an active treatment in previously treated HER2-positive mBC patients. However, only limited data exist on the efficacy of trastuzumab emtansine in patients who were previously treated with the combination of both trastuzumab and pertuzumab in combination with a taxane, the first-line treatment that eventually became standard-of-care in this setting (3). Only a small retrospective study with trastuzumab emtansine in patients previously treated with pertuzumab suggested a clinically relevant benefit based on prolonged duration of therapy and objective response rate (9). However, 48% of these patients received trastuzumab emtansine as fourth-line treatment or later and not as second line. Interestingly, response rate and progression-free survival with trastuzumab emtansine after previous treatment with pertuzumab were lower than those reported from others studies of trastuzumab emtansine in patients previously treated with HER2-directed therapy without pertuzumab (4,5).
Full table
If the confirmation of the overall survival improvement with trastuzumab emtansine reported in EMILIA clearly strengthens its position as the reference for second-line treatment in HER2-positive mBC, the final overall survival analysis of TH3RESA is more challenging to interpret. The primary analysis of TH3RESA study, published in 2014, showed that trastuzumab emtansine was associated with a statistically significant improvement in progression-free survival [median 6.2 vs. 3.3 months; hazard ratio 0.528 (95% CI, 0.422–0.661)], and a more favorable safety profile relative to treatment of physician’s choice in heavily pretreated HER2-positive mBC patients (5). In the final analysis of overall survival of TH3RESA, trastuzumab emtansine conferred statistically and clinically significant improvements in overall survival versus treatment of physician’s choice [median 22.7 vs. 15.8 months; hazard ratio 0.68 (95% CI, 0.54–0.85)] (8). This survival advantage remained despite a substantial—not preplanned—cross over of patients from the control arm to trastuzumab emtansine (47%) that was eventually permitted after the first overall survival interim analysis was reported. Thus, the overall survival results of TH3RESA might suggest the relevance of using trastuzumab emtansine in the treatment of HER2-positive mBC after several previous treatments. However, it remains to be clarified whether trastuzumab emtansine should be used as early as possible (in second line), or later in the disease as salvage treatment when all HER2-targeting agents have been exhausted. In the management of metastatic cancer patients, it is challenging not to give the presumably best possible treatment option and to preserve it for an uncertain future, especially in the absence of reliable prognostic and predictive factors. As previously mentioned, HER2-positive breast cancer is a biologically aggressive disease, as exemplified by the fact that nearly 25% of patients who discontinued lapatinib and capecitabine in EMILIA and 20% in the control arm of TH3RESA had no further treatment, likely because of clinical deterioration (7,8). Moreover, the fact that 61% of patients in EMILIA were treated with trastuzumab emtansine at the first disease progression after a trastuzumab-based first-line of treatment strongly supports an early use of trastuzumab emtansine (7). In addition, trastuzumab emtansine has a clearly better safety profile compared to the control treatments in EMILIA and TH3RESA, with fewer grade 3 or more adverse events (Table 1) (7,8). In TH3RESA, the favorable safety profile was maintained, even though the mean treatment duration was nearly twice as long for patients treated with trastuzumab emtansine compared with patients treated with the treatment at physician’s choice, who were mainly receiving trastuzumab-based treatment regimens (8). After an adjustment for the longer duration of treatment exposure in patients treated with trastuzumab emtansine, it was associated with less than half of grade 3 or more adverse events (123.6 vs. 278.4 events per 100 patient-years) (8). Even if not further practice-changing, the final overall survival results of TH3RESA have at least two major practical implications. The first is the confirmation of the central role played by HER2 amplification in mBC, even in heavily pretreated patients. Since only 17% of patients in the control arm were treated with a non-HER2-targeting treatment regimen, the study cannot be considered as a comparative trial between continuing or not HER2 blockade in patients with trastuzumab-resistant metastatic disease. However, the overall survival improvement obtained with trastuzumab emtansine even in the remaining 83% of patients treated with a combination with an HER2-directed agent (trastuzumab plus chemotherapy, trastuzumab plus lapatinib, trastuzumab plus hormone therapy or lapatinib plus chemotherapy), as confirmed by the overall survival analysis specific to this subgroup (8), strongly underlines the fact that not all HER2-targeted therapies are equal. Even if the HER2 pathway is clearly crucial, including in later lines of therapy, the way to inhibit HER2 is even more important. The second aspect is directly linked to the first, since these results further imply a critical analysis about the best way to set up clinical trials that will evaluate the new HER-2 targeted therapies currently in development (such as different HER2-directed monoclonal antibodies and tyrosine kinase inhibitors, novel approaches conjugating HER2 antibodies with various toxic payloads, or combining HER2 antibodies with cellular immunotherapy) (10): it will be essential to avoid the trap of using too strict therapeutic sequences as reference, since they probably have more a practical than a biological value.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. [Crossref] [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [Crossref] [PubMed]
- Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109-19. [Crossref] [PubMed]
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91. [Crossref] [PubMed]
- Krop IE, Kim SB, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:689-99. [Crossref] [PubMed]
- Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol 2017;28:16-33. [Crossref] [PubMed]
- Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:732-42. [Crossref] [PubMed]
- Krop IE, Kim SB, Martin AG, et al. Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol 2017;18:743-54. [Crossref] [PubMed]
- Dzimitrowicz H, Berger M, Vargo C, et al. T-DM1 Activity in Metastatic Human Epidermal Growth Factor Receptor 2-Positive Breast Cancers That Received Prior Therapy With Trastuzumab and Pertuzumab. J Clin Oncol 2016;34:3511-7. [Crossref] [PubMed]
- Parakh S, Gan HK, Parslow AC, et al. Evolution of anti-HER2 therapies for cancer treatment. Cancer Treat Rev 2017;59:1-21. [Crossref] [PubMed]


