Chimeric antigen receptor (CAR) T and other T cell strategies for pancreas adenocarcinoma
Introduction
Pancreas cancer represents the third highest cause of cancer-related death in the United States and will affect an anticipated 53,670 patients (1). The majority of patients with pancreas cancer are diagnosed with metastatic disease, and treatments are based in systemic chemotherapy. With the approval of new chemotherapy regimens, including the 5-fluorouracil-based combination with irinotecan and oxaliplatin (FOLFIRINOX) and the combination of gemcitabine and albumin-bound paclitaxel, several reports note overall survival measuring up to 2 years and greater with systemic therapy alone (2-6). While these benefits are tangible for many patients, the improvements with systemic chemotherapy remain incremental. Immunotherapy has changed the way we treat metastatic disease in many solid tumors, including melanoma and lung cancer, bringing durable responses to many of these patients (7-9). To understand whether immunotherapy has a role in the treatment paradigm of pancreas cancer, many ongoing studies are evaluating the following agents as single agents and in combination: immune checkpoint inhibitors, small molecule inhibitors, stromal modulating agents, and genetically engineered and modified T cells. Genetically modified T cells are changing the way we approach and treat hematologic malignancies, and much research is ongoing to understand if this technology can be used to treat solid tumors as well. The focus of this review is to describe the underlying biology of T cell therapy and ongoing T cell therapy studies in pancreas cancer.
Adoptive T cell transfer
Adoptive T cell therapy exploits the patient’s own immune system. After a patient’s own T cells are harvested, the T cell receptor can be modified to recognize a specific tumor antigen, expanded, and then reinfused into a patient, allowing the patient’s own T cell to target a specified antigen within the context of the patient’s own self major histocompatibility complex (MHC). T cells comprise a critical arm of the immune system to identify and adapt to non-self-antigens and targets. Through identification of non-self-antigens, T cell receptors are activated and then stimulated to proliferate, develop into effector cells, and initiate cytolysis against the target cell (10). T cell receptors are comprised of an alpha and beta heterodimer to recognize non-self-antigen presented by the matched MHC and are further stimulated through the associated CD3 complex composed of the zeta-zeta homodimer and heterodimers of epsilon-gamma and epsilon-delta (10).
This adoptive T cell therapy demonstrated promise in both preclinical and clinical studies using T cells targeting MUC1, an antigen present on pancreas cancer cells, in an HLA-independent fashion. In one study, MUC1-specific T cells were generated via exposure and stimulation of a patient’s harvested T cells with a pancreas cancer cell line expressing MUC1. These MUC1-targeted T cells demonstrated in vitro activity against multiple pancreas cancer cell lines (11). In a related study in which 20 patients with unresectable or recurrent pancreas cancer were treated with these MUC1-targeted T cells, five experienced stable disease, and one patient experienced a complete response. Mean survival time was 9.8 months (12).
First generation chimeric antigen receptor T cells
In an effort to avoid the MHC restriction needed for conventional T cell receptor activation, T cells can be transduced with genes encoding a chimeric antigen receptor (CAR), which includes both an extracellular binding domain and an intracellular activation domain often using the CD3 T cell activation domain. This extracellular binding domain includes an antibody recognizing tumor antigens, thus avoiding the MHC restriction needed for conventional T cells and allowing for the engineering of CAR T cells to express receptors specific for many different tumor antigens (10). By transducing both a CAR and a T cell activation domain, these first-generation CAR T cells identify and attack tumor antigens and also expand with the help of T cell activation through the CD3 complex. CAR T cells are then infused back into a patient to stimulate an immune response against the target antigen. Additionally, tumor lysis may result in cytokine production, further stimulating an immune response (13). Although first generation CAR T cells have shown some ability to persist and cause anti-tumor activity, initial studies demonstrate that anti-tumor activity, expansion, and persistence are sub-optimal (14-16). To combat these challenges, first generation CAR T cells have been modified with the addition of co-stimulatory molecules to create so-called second-generation CAR T cells.
Second generation chimeric antigen receptor T cells
Second generation CAR T cells are further modified with the addition of a second co-stimulatory cytoplasmic signaling domain such as 41-BB (also known as CD137), OX40 (also known as CD134), CD28, inducible costimulator (ICOS), and DAP10 (17-28). Stimulation of these costimulatory intracytoplasmic domains with CAR activation by tumor antigen allows production of IL-2 and other stimulatory cytokines to facilitate activation and expansion of these CAR T cell populations, as demonstrated pre-clinically through increased IL-2 production after antigen binding to a CD28-derived-CD3 zeta CAR compared to decreased IL-2 production with antigen binding to a CAR T cell without CD28 (27,29-31).
Promising responses in CD19 expressing hematologic malignancies
Thus far, CAR T cells have been evaluated most extensively in CD19 expressing B cell malignancies with most promise in B-cell acute lymphoblastic leukemia (B-ALL). The largest experience to date in adults has been reported by Park and colleagues in which 37/45 patients experienced morphologic complete response, and 13/37 were able to undergo an allogeneic hematopoietic stem cell transplant. Six-month overall survival did not differ between patients who did or did not undergo allogeneic hematopoietic stem cell transplant. Most common toxicities in this group included transient B-cell aplasia, cytokine release syndrome (CRS), and neurologic toxicities (32-34). At Fred Hutchinson Cancer Research Center, 24/26 evaluable patients with B-ALL achieved a complete response (35).
CD19-targeted CAR T cells have shown promise in pediatric B-ALL studies as well. Investigators from University of Pennsylvania have demonstrated that 50/53 patients experienced a morphologic complete response (36,37). Investigators from the National Cancer Institute have shown that 14/20 patients with relapsed B-ALL achieved and maintained a complete response as well (38). At Memorial Sloan Kettering Cancer Center, initial results have demonstrated that 2/4 patients with relapsed B-ALL experienced a complete response with treatment with CD19-targeted CAR T cells (39).
Many ongoing studies are evaluating the efficacy of CAR T cells in patients with other CD19 expressing B cell malignancies, including multiple myeloma, non-Hodgkin lymphoma, and chronic lymphocytic leukemia (40). Recently completed studies evaluating CAR T cells in other CD19 B cell malignancies have demonstrated mixed responses (40,41).
Emerging data and experience in solid tumors
Given the promising responses seen with CAR T cells in CD19 expressing hematologic malignancies, efforts are underway to understand whether this emerging technology can be applied to solid tumors. CAR T cells have been created to target many tumor antigens in various solid tumors including pancreas cancer, mesothelioma, ovarian cancer, renal cell carcinoma, and melanoma (15,42-47). Although few studies with adoptive T cell therapy in pancreas cancer have been completed thus far, ongoing studies for patients with pancreas cancer are evaluating many tumor antigens, such as carcinoembryonic antigen (CEA), mesothelin, and others as described below.
CEA
Routinely used as a serum marker for monitoring patients with gastrointestinal malignancies, CEA is also present on the tumor cell surface of 75% of pancreas cancers (48-50). Preclinical studies have demonstrated that CAR T cells targeting CEA have a cytotoxic effect on a pancreas cancer mouse model (51). Although final results have not yet been published, a CAR T cell study evaluating CEA-targeted CAR T cells was halted early due to respiratory complications, highlighting the risks and caution needed when pursuing these studies (52,53). Several studies are ongoing to better understand the safety and maximum tolerated dose of CAR T cells targeting CEA through both systemic administration (NCT02349724 Southwest Hospital China) and various local means of administration to the liver (NCT02850536 Roger Williams Medical Center, NCT 02959151 Shanghai Cancer Hospital).
Mesothelin
Mesothelin is expressed in many tumors, including 85–90% of mesotheliomas and 80–85% of pancreas cancers (54-57). Mesothelin’s utility as a practical target for immune targeting was confirmed in a phase 1 study in which an anti-mesothelin immunotoxin SS1P was administered to patients with mesothelin-expressing tumors (58). Of 33 patients treated, 23 experienced disease control with 4 patients having a minor response and 19 patients having stable disease (58). In pancreas cancer, expression of mesothelin is present primarily in pancreas adenocarcinomas but not in normal tissue (59,60). Mesothelin is associated with tumor invasion (59,60). In preclinical studies, CAR T cells targeting mesothelin demonstrated response in pancreas cancer cell lines (61).
Preliminary results presented by Beatty and colleagues demonstrated the feasibility of mesothelin-specific CAR T cell therapy for patients with solid tumors, including 1 with metastatic pancreas cancer (62). Follow-up data noted that 2 out of 6 patients experienced stable disease, including 1 with stable disease for greater than 4 months while off cytotoxic chemotherapy (63) (NCT01897415 University of Pennsylvania). Final results are pending.
Many ongoing studies are evaluating the role of mesothelin-targeted CAR T cells in pancreas cancer (NCT01583686 National Cancer Institute, NCT02159716 University of Pennsylvania, NCT02465983 University of Pennsylvania/University of California, San Francisco, NCT03054298 University of Pennsylvania, NCT02930993 China Meitan General Hospital, NCT 02580747 Chinese PLA General Hospital). Novel approaches of locally administered CAR T cells are also being further investigated (NCT02706782, Renji Hospital in China, NCT01897415, NCT02706782, NCT02959151 Shanghai Cancer Hospital). Additional studies are also ongoing evaluating the role of mesothelin-targeted CAR T cells in mesothelioma and in breast cancer with pleural involvement (NCT02414269 Memorial Sloan Kettering Cancer Center, NCT02792114 Memorial Sloan Kettering Cancer Center).
Prostate stem cell antigen
Harnessing the overexpression of prostate stem cell antigen (PSCA) in pancreas cancer, PSCA-targeted CAR T cells demonstrated promise in an in vivo mouse model of pancreas cancer, supporting further evaluation of this target (64,65). A novel CAR T cell construct, BPX-601, has been developed with a variable chain fragment targeting PSCA and with an inducible co-stimulatory domain activated by the small molecule rimiducid. The safety of BPX-601 is being evaluated in an ongoing clinical trial (NCT02744287 Bellicum Pharmaceuticals).
Other antigens
CD24
CD24, a marker of cancer stem cells, is also being evaluated as a therapeutic target in pancreas cancer. Given the prevalence of early disseminated metastases and the treatment refractory nature of this disease, cancer stem cells are thought to play a role in the development and proliferation of pancreas cancer (48,66). Preclinical studies have demonstrated that CD24-targeted CAR T cells caused tumor eradication in a xenograft model of pancreas cancer (67).
MUC1
MUC1 is overexpressed in pancreas cancer, and preclinical studies have demonstrated promising responses in vivo studies of MUC1-targeted CAR T cells in mice (68,69). A case report was presented of minimal toxicity and tumor necrosis after intra-tumoral administration of MUC1-targeted CAR T cells in a patient with MUC1-expressing seminal vesicle cancer (70). Studies are ongoing to evaluate the safety and efficacy of MUC1-targeted CAR T cells (NCT02587689 PersonGen BioTherapeutics Co., Ltd., NCT02617134 PersonGen BioTheraputics Co,. Ltd.).
MUC16
MUC16, also known as CA125, is a well-known marker in gynecologic malignancies and is also overexpressed in pancreas cancer cell lines and not expressed in normal pancreas (71). In vitro studies have demonstrated antitumor activity and eradication in a mouse model of peritoneal cancer treated with MUC16-targeted CAR T cells targeting MUC16 (72). Studies are ongoing evaluating the safety and maximum tolerated dose of MUC16-targeted CAR T cells in patients with gynecologic cancers (73).
Many studies are ongoing for patients with pancreas cancer evaluating CAR T cells in pancreas cancer against various other targets, including CD133, CD70, epidermal growth factor receptor (EGFR), ERBB2, natural killer receptors, and others (Table 1).
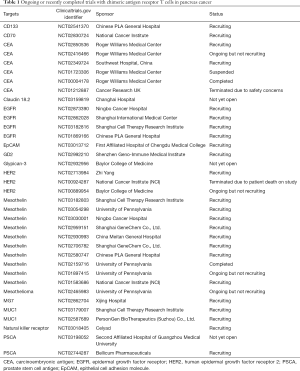
Full table
Challenges and opportunities in pancreas cancer
Stromal modulation
Several features of pancreas cancer contribute to the chemoresistance and poor prognosis of pancreas cancer. One such feature is the dense stroma surrounding pancreas cancer, which is thought to limit chemotherapy delivery to the tumor. The stroma itself represents a promising therapeutic target to not only facilitate chemotherapy delivery but also to propagate a more favorable tumor microenvironment, and many studies are ongoing or have been recently completed to evaluate the role of stromal targeting in this population. For instance, a recent study of the combination of chemotherapy and PEGPH20 targeting hyaluronidase demonstrated promising activity, and several studies are ongoing evaluating this combination (NCT02715804, NCT02487277, NCT 02921022) (74,75). Heparanase was also a promising target, although a recently completed study of the heparanase targeted drug M402 with gemcitabine and nab-paclitaxel was recently stopped early due to futility (76). Chemokine receptors, such as CXCR2, have also been a promising target. A recent study of PF04136309, a CXCR2 antagonist, with FOLFIRINOX demonstrated promising results, and CAR T cells co-expressing chemokine receptors are being explored to enhance CAR T cell trafficking to the tumor (77-79). Building on these strategies, several CAR T cell studies are also targeting the tumor stroma. For instance, fibroblast activation protein (FAP) is expressed on stromal cells, and FAP-targeted CAR T cells have been developed and demonstrated tumor activity in mouse models but also off-tumor on-target toxicity, which may limit further evaluation (80).
Immunosuppressive tumor microenvironment
Another challenge in pancreas cancer is the creation of an immunosuppressive tumor microenvironment that allows pancreas cancer to evade the immune system. First, pancreas cancer is thought to harbor an increased number of T regulatory cell populations, partly via increased expression of chemokine receptor type 5 (CCR5). Increased presence of T regulatory cells is thought to contribute to T cell inhibition via increased inhibitory cytokine production such as TGF-beta and IL-10 (48,81,82). Also, upregulation of inhibitory ligands such as programmed death-1 (PD-1) results in T cell inhibition. Single agent use of immune checkpoint inhibitors has resulted in durable and dramatic clinical response in many cancers, including lung cancer, melanoma, renal cell carcinoma, and others. While single agent use of checkpoint blockade has been underwhelming in pancreas cancer thus far, use of checkpoint blockade in combination with CAR T cells may circumvent these issues (83). To augment the response of CAR T cells, combination therapy with immune checkpoint blockade and CAR T cells has demonstrated restored and prolonged efficacy of CAR T cells (84). Consequently, novel combinations of immune checkpoint blockade with mesothelin-targeted CAR T cells (NCT03182803 Shanghai Cell Therapy Research Institute, NCT03030001 Ningbo Cancer Hospital), MUC1 expressing CAR T cells (NCT03179008 Shanghai Cell Therapy Research Institute) are also ongoing.
Another approach has been to further modify CAR T cells to thrive in an immunosuppressive environment. Mohammed and colleagues evaluated the role of the immunosuppressive cytokine IL-4. They created a molecule combining the IL-4 receptor to the endodomain of the immune-activating IL-7 receptor. Transduction of this molecule into a PSCA-targeted CAR T cell allowed the CAR T cell to persist and thrive in an IL-4 rich microenvironment using an IL-4 producing prostate stem cell antigen (PSCA)-expressing tumor cell line (85).
Similarly, use of other immune modulatory agents such as tumor necrosis factor alpha-receptor antibodies and diabetes medication such as metformin and rosiglitazone are novel therapeutic approaches and may provide opportunities to be combined with CAR T cell to facilitate improved activity of CAR T cell (86-88).
Safety concerns with chimeric antigen receptor T cells
While CAR T cell therapy has provided much excitement in the hematologic malignancies and promise in solid tumors, several drawbacks and concerning toxicities have also arisen. The biggest challenge in application of CAR T cell technology to solid tumors is that antigens present on the tumor cell surface are nearly always present in some level on normal tissue as well, increasing risk of autoimmune on-target toxicity. Also, there have been several treatment-related deaths on clinical trials using CAR T cells in hematologic malignancies, related to neurologic toxicity and cerebral edema (89). Finally, CRS remains a significant concern and requires monitoring with an experienced team and frequently monitoring in an intensive care unit setting due to the risks of rapid progression and decline (41,90).
Many strategies have been developed to help monitor and treat these side effects, particularly among centers treating a higher volume of these patients. First, transduction of a “suicide” gene into the CAR T cell is often used. For instance, transduction of the epidermal growth factor receptor into the CAR T cell allows for apoptosis with administration of cetuximab, an FDA-approved drug targeting EGFR which can be well-tolerated and with a well-known safety profile. Use of the IL-6 receptor antagonist tocilizumab is also used to help treat CRS. Most important though is a high degree of suspicion for toxicity, early intervention, and appropriate use of reversal mechanisms such as tocilizumab or triggers for the suicide gene used in that CAR T cell construct (41,90).
Future directions
Pancreas cancer remains a disease treated by systemic chemotherapy primarily, and novel treatment strategies are needed. Immunotherapy in the form of checkpoint inhibitors has revolutionized the way we treat lung cancer, melanoma, and several others, and T cell therapy has similarly provided dramatic and frequently durable improvements in a challenging population of patients with refractory CD19+ hematologic malignancies. We remain hopeful that T cell therapies will provide a novel opportunity for patients with pancreas cancer given the multitude of targets and opportunities for both local administration of CAR T cells and combination therapy with other immunotherapies and stromal modulating agents.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Portal A, Pernot S, Arbaud C, et al. Nab paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after failure of folfirinox: Results of an AGEO multicenter prospective cohort. J Clin Oncol 2015;33:4123. [PubMed]
- Ramanathan RK, Lee P, Seng JE, et al. Phase II study of induction therapy with gemcitabine and nab-paclitaxel followed by consolidation with mFOLFIRINOX in patients with metastatic pancreatic cancer. J Clin Oncol 2014;32:224. [Crossref]
- Goldstein D, Chiorean EG, Tabernero J, et al. Outcome of second-line treatment (2L Tx) following nab-paclitaxel (nab-P) + gemcitabine (G) or G alone for metastatic pancreatic cancer (MPC). J Clin Oncol 2016;34:333. [Crossref]
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer 2003;3:35-45. [Crossref] [PubMed]
- Kawaoka T, Oka M, Takashima M, et al. Adoptive immunotherapy for pancreatic cancer: cytotoxic T lymphocytes stimulated by the MUC1-expressing human pancreatic cancer cell line YPK-1. Oncol Rep 2008;20:155-63. [PubMed]
- Kondo H, Hazama S, Kawaoka T, et al. Adoptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res 2008;28:379-87. [PubMed]
- Gong MC, Latouche JB, Krause A, et al. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia 1999;1:123-7. [Crossref] [PubMed]
- Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant 2010;16:1245-56. [Crossref] [PubMed]
- Lamers CH, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol 2006;24:e20-2. [Crossref] [PubMed]
- Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 2008;112:2261-71. [Crossref] [PubMed]
- Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res 2007;13:5426-35. [Crossref] [PubMed]
- Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol 2004;172:104-13. [Crossref] [PubMed]
- Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004;18:676-84. [Crossref] [PubMed]
- Pule MA, Straathof KC, Dotti G, et al. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther 2005;12:933-41. [Crossref] [PubMed]
- Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011;121:1822-6. [Crossref] [PubMed]
- Teng MW, Kershaw MH, Moeller M, et al. Immunotherapy of cancer using systemically delivered gene-modified human T lymphocytes. Hum Gene Ther 2004;15:699-708. [Crossref] [PubMed]
- Sasaki T, Fujimori M, Hamaji Y, et al. Genetically engineered Bifidobacterium longum for tumor-targeting enzyme-prodrug therapy of autochthonous mammary tumors in rats. Cancer Sci 2006;97:649-57. [Crossref] [PubMed]
- Teng MW, Kershaw MH, Jackson JT, et al. Adoptive transfer of chimeric FcepsilonRI gene-modified human T cells for cancer immunotherapy. Hum Gene Ther 2006;17:1134-43. [Crossref] [PubMed]
- Gyobu H, Tsuji T, Suzuki Y, et al. Generation and targeting of human tumor-specific Tc1 and Th1 cells transduced with a lentivirus containing a chimeric immunoglobulin T-cell receptor. Cancer Res 2004;64:1490-5. [Crossref] [PubMed]
- Haynes NM, Trapani JA, Teng MW, et al. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood 2002;100:3155-63. [Crossref] [PubMed]
- Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol 2002;20:70-5. [Crossref] [PubMed]
- Westwood JA, Smyth MJ, Teng MW, et al. Adoptive transfer of T cells modified with a humanized chimeric receptor gene inhibits growth of Lewis-Y-expressing tumors in mice. Proc Natl Acad Sci U S A 2005;102:19051-6. [Crossref] [PubMed]
- Hombach A, Sent D, Schneider C, et al. T-cell activation by recombinant receptors: CD28 costimulation is required for interleukin 2 secretion and receptor-mediated T-cell proliferation but does not affect receptor-mediated target cell lysis. Cancer Res 2001;61:1976-82. [PubMed]
- Geiger TL, Nguyen P, Leitenberg D, et al. Integrated src kinase and costimulatory activity enhances signal transduction through single-chain chimeric receptors in T lymphocytes. Blood 2001;98:2364-71. [Crossref] [PubMed]
- Finney HM, Lawson AD, Bebbington CR, et al. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol 1998;161:2791-7. [PubMed]
- Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5:177ra38. [Crossref] [PubMed]
- Park JH, Riviere I, Wang X, et al. Efficacy and safety of CD19-targeted 19-28z CAR modified T cells in adult patients with relapsed or refractory B-ALL. J Clin Oncol 2015;33:7010.
- Park JH, Riviere I, Wang X, et al. Implications of minimal residual disease negative complete remission (MRD-CR) and allogeneic stem cell transplatn on safety and clinical outcome of CD19-targeted 19-28z CAR modified T cells in adult patients with relapsed, refractory B-cell ALL. Blood 2015;126:682.
- Turtle CJ, Hanafi LA, Berger C, et al. Addition of fludarabine to cyclophosphamide lymphodepletion improves in vivo expansion of CD19 chimeric antigen receptor-modified T cells and clinical outcome in adults with B cell acute lymphoblastic leukemia. Blood 2015.126.
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Grupp SA, Maude SL, Shaw PA, et al. Durable Remissions in Children with Relapsed/Refractory ALL Treated with T Cells Engineered with a CD19-Targeted Chimeric Antigen Receptor (CTL019). Blood 2015;126:681.
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517-28. [Crossref] [PubMed]
- Curran K, Riviere I, Kobos R, et al. Chimeric Antigen Receptor (CAR) T Cells Targeting the CD19 Antigen for the Treatment of Pediatric Relapsed B Cell ALL. Blood 2014;124:3716.
- Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood 2016;127:3312-20. [Crossref] [PubMed]
- Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat Rev Clin Oncol 2016;13:370-83. [Crossref] [PubMed]
- Altenschmidt U, Kahl R, Moritz D, et al. Cytolysis of tumor cells expressing the Neu/erbB-2, erbB-3, and erbB-4 receptors by genetically targeted naive T lymphocytes. Clin Cancer Res 1996;2:1001-8. [PubMed]
- Moritz D, Wels W, Mattern J, et al. Cytotoxic T lymphocytes with a grafted recognition specificity for ERBB2-expressing tumor cells. Proc Natl Acad Sci U S A 1994;91:4318-22. [Crossref] [PubMed]
- Darcy PK, Haynes NM, Snook MB, et al. Redirected perforin-dependent lysis of colon carcinoma by ex vivo genetically engineered CTL. J Immunol 2000;164:3705-12. [Crossref] [PubMed]
- Haynes NM, Snook MB, Trapani JA, et al. Redirecting mouse CTL against colon carcinoma: superior signaling efficacy of single-chain variable domain chimeras containing TCR-zeta vs Fc epsilon RI-gamma. J Immunol 2001;166:182-7. [Crossref] [PubMed]
- Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 2006;12:6106-15. [Crossref] [PubMed]
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62-8. [Crossref] [PubMed]
- DeSelm CJ, Tano ZE, Varghese AM, et al. CAR T-cell therapy for pancreatic cancer. J Surg Oncol 2017;116:63-74. [Crossref] [PubMed]
- Albers GH, Fleuren G, Escribano MJ, et al. Immunohistochemistry of CEA in the human pancreas during development, in the adult, chronic pancreatitis, and pancreatic adenocarcinoma. Am J Clin Pathol 1988;90:17-22. [Crossref] [PubMed]
- Allum WH, Stokes HJ, Macdonald F, et al. Demonstration of carcinoembryonic antigen (CEA) expression in normal, chronically inflamed, and malignant pancreatic tissue by immunohistochemistry. J Clin Pathol 1986;39:610-4. [Crossref] [PubMed]
- Chmielewski M, Hahn O, Rappl G, et al. T cells that target carcinoembryonic antigen eradicate orthotopic pancreatic carcinomas without inducing autoimmune colitis in mice. Gastroenterology 2012;143:1095-107.e2. [Crossref] [PubMed]
- Gilham DE, Anderson J, Bridgeman JS, et al. Adoptive T-cell therapy for cancer in the United kingdom: a review of activity for the British Society of Gene and Cell Therapy annual meeting 2015. Hum Gene Ther 2015;26:276-85. [Crossref] [PubMed]
- Thistlethwaite F, Guest R, Rothwell D, et al. A CRUK Phase 1 trial of adoptive transfer of autologous tumour antigen-specific T cells with pre-conditioning chemotherapy and intravenous IL2 in patients with advanced CEA positive tumours. NCRI Poster Session 2010.
- Morello A, Sadelain M, Adusumilli PS. Mesothelin-Targeted CARs: Driving T Cells to Solid Tumors. Cancer Discov 2016;6:133-46. [Crossref] [PubMed]
- Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res 2001;7:3862-8. [PubMed]
- Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A 1996;93:136-40. [Crossref] [PubMed]
- Pastan I, Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res 2014;74:2907-12. [Crossref] [PubMed]
- Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res 2007;13:5144-9. [Crossref] [PubMed]
- Shimizu A, Hirono S, Tani M, et al. Coexpression of MUC16 and mesothelin is related to the invasion process in pancreatic ductal adenocarcinoma. Cancer Sci 2012;103:739-46. [Crossref] [PubMed]
- Hassan R, Laszik ZG, Lerner M, et al. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol 2005;124:838-45. [Crossref] [PubMed]
- Stromnes IM, Schmitt TM, Hulbert A, et al. T Cells Engineered against a Native Antigen Can Surmount Immunologic and Physical Barriers to Treat Pancreatic Ductal Adenocarcinoma. Cancer Cell 2015;28:638-52. [Crossref] [PubMed]
- Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2014;2:112-20. [Crossref] [PubMed]
- Beatty GL, O’Hara MH, Nelson AM, et al. Safety and antitumor activity of chimeric antigen receptor modified T cells in patients with chemotherapy refractory metastatic pancreatic cancer. J Clin Oncol 2015;33:3007.
- Abate-Daga D, Lagisetty KH, Tran E, et al. A novel chimeric antigen receptor against prostate stem cell antigen mediates tumor destruction in a humanized mouse model of pancreatic cancer. Hum Gene Ther 2014;25:1003-12. [Crossref] [PubMed]
- Katari UL, Keirnan JM, Worth AC, et al. Engineered T cells for pancreatic cancer treatment. HPB (Oxford) 2011;13:643-50. [Crossref] [PubMed]
- Sagiv E, Kazanov D, Arber N. CD24 plays an important role in the carcinogenesis process of the pancreas. Biomed Pharmacother 2006;60:280-4. [Crossref] [PubMed]
- Maliar A, Servais C, Waks T, et al. Redirected T cells that target pancreatic adenocarcinoma antigens eliminate tumors and metastases in mice. Gastroenterology 2012;143:1375-84.e1-5.
- Qu CF, Li Y, Song YJ, et al. MUC1 expression in primary and metastatic pancreatic cancer cells for in vitro treatment by (213)Bi-C595 radioimmunoconjugate. Br J Cancer 2004;91:2086-93. [Crossref] [PubMed]
- Posey AD Jr, Schwab RD, Boesteanu AC, et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity 2016;44:1444-54. [Crossref] [PubMed]
- You F, Jiang L, Zhang B, et al. Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified Anti-MUC1 chimeric antigen receptor transduced T cells. Sci China Life Sci 2016;59:386-97. [Crossref] [PubMed]
- Haridas D, Chakraborty S, Ponnusamy MP, et al. Pathobiological implications of MUC16 expression in pancreatic cancer. PLoS One 2011;6:e26839. [Crossref] [PubMed]
- Chekmasova AA, Rao TD, Nikhamin Y, et al. Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin Cancer Res 2010;16:3594-606. [Crossref] [PubMed]
- Koneru M, O'Cearbhaill R, Pendharkar S, et al. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med 2015;13:102. [Crossref] [PubMed]
- Hingorani SR, Harris WP, Hendifar AE, et al. High response rate and PFS with PEGPH20 added to nab-paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients with high-HA tumors: Interim results of a randomized phase II study. J Clin Oncol 2015;33:4006. [Crossref]
- Hingorani SR, Bullock AJ, Seery TE, et al. Randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine (PAG) vs AG in patients (Pts) with untreated, metastatic pancreatic ductal adenocarcinoma (mPDA). J Clin Oncol 2017;35:4008.
- Momenta Discontinues Further Accrual of its Phase 2 Trial of Necuparanib in Patients with Pancreatic Cancer Following Planned Interim Futility Analysis. 2016. Available online: https://globenewswire.com/news-release/2016/08/04/861680/0/en/Momenta-Discontinues-Further-Accrual-of-its-Phase-2-Trial-of-Necuparanib-in-Patients-with-Pancreatic-Cancer-Following-Planned-Interim-Futility-Analysis.html
- Caruana I, Savoldo B, Hoyos V, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med 2015;21:524-9. [Crossref] [PubMed]
- Craddock JA, Lu A, Bear A, et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother 2010;33:780-8. [Crossref] [PubMed]
- Moon EK, Carpenito C, Sun J, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res 2011;17:4719-30. [Crossref] [PubMed]
- Tran E, Chinnasamy D, Yu Z, et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med 2013;210:1125-35. [Crossref] [PubMed]
- Shibuya KC, Goel VK, Xiong W, et al. Pancreatic ductal adenocarcinoma contains an effector and regulatory immune cell infiltrate that is altered by multimodal neoadjuvant treatment. PLoS One 2014;9:e96565. [Crossref] [PubMed]
- Tan MC, Goedegebuure PS, Belt BA, et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol 2009;182:1746-55. [Crossref] [PubMed]
- Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010;33:828-33. [Crossref] [PubMed]
- Cherkassky L, Morello A, Villena-Vargas J, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 2016;126:3130-44. [Crossref] [PubMed]
- Mohammed S, Sukumaran S, Bajgain P, et al. Improving Chimeric Antigen Receptor-Modified T Cell Function by Reversing the Immunosuppressive Tumor Microenvironment of Pancreatic Cancer. Mol Ther 2017;25:249-58. [Crossref] [PubMed]
- Bunt SK, Mohr AM, Bailey JM, et al. Rosiglitazone and Gemcitabine in combination reduces immune suppression and modulates T cell populations in pancreatic cancer. Cancer Immunol Immunother 2013;62:225-36. [Crossref] [PubMed]
- Eikawa S, Nishida M, Mizukami S, et al. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A 2015;112:1809-14. [Crossref] [PubMed]
- Aida K, Miyakawa R, Suzuki K, et al. Suppression of Tregs by anti-glucocorticoid induced TNF receptor antibody enhances the antitumor immunity of interferon-alpha gene therapy for pancreatic cancer. Cancer Sci 2014;105:159-67. [Crossref] [PubMed]
- Juno Therapeutics reports fourth quarter and 2016 financial results. 2017. Available online: http://ir.junotherapeutics.com/phoenix.zhtml?c=253828&p=irol-newsArticle&ID=2250772
- Maude SL, Barrett D, Teachey DT, et al. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J 2014;20:119-22. [Crossref] [PubMed]


