Adenocarcinoma of the esophagus: controversies and consensus
Introduction
Esophageal cancer is an uncommon disease in the West, and will be diagnosed in 16,940 patients and account for 15,690 deaths in the US in 2017 (1). Squamous cell cancer is the more common histology globally, whereas denocarcinoma is the most common histology in Western countries, related to reflux-associated Barrett’s esophagus. An ongoing increase in adenocarcinoma of the distal esophagus and gastroesophageal junction (GEJ) has occurred over the past 4 decades (2,3). With nearly 500,000 cases seen annually around the world, esophageal cancer is the sixth leading cause of cancer related death (4). Metastatic disease is present in half of patients at diagnosis, and patients with local regional disease develop metastatic disease in the majority of cases. Given the anatomy of the mediastinum and GEJ, the risk of incomplete surgical resection and local tumor recurrence is significant. Neoadjuvant or preoperative therapy is now routinely included given the poor outcomes with surgical management alone.
Surgical treatment of esophageal cancer
Resection of the esophagus with en bloc lymphadenectomy is the cornerstone of curative treatment for patients with locally advanced esophageal cancer (5). Treatment in high-volume centers with experienced surgeons and the availability of critical-care support are associated with improved outcomes and lower morbidity and mortality (6,7).
A transthoracic esophagectomy with two-field lymph node dissection and gastric conduit reconstruction is the preferred procedure for resection of esophageal cancer (8,9). Transhiatal esophagectomy, without thoracotomy was designed to reduce postoperative morbidity and mortality by avoiding thoracotomy (10,11). A randomized clinical trial, comparing an extended transthoracic resection with a transhiatal approach showed that transhiatal esophagectomy was associated with lower morbidity and shorter intensive care unit and hospital stay compared to transthoracic esophagectomy (12). Compared with transhiatal resection, transthoracic esophagectomy for esophageal adenocarcinoma indicated a trend towards better 5-year survival (13,14). Minimally invasive esophagectomy, and more recently robotically assisted esophagectomy, are emerging as surgical alternatives to open esophagectomy (15-18).
For clinical T2N0 esophageal cancer, surgery alone is regarded as a standard treatment approach without neoadjuvant therapy (19-21). Current preoperative staging of patients with T2N0 esophageal cancer may lead to clinical understaging in a substantial proportion of patients (22-24). In addition, trials have not confirmed a clear survival benefit of a preoperative therapy compared to a surgery alone for T2N0 esophageal cancer (21,25-28). There is on-going controversy about treating these patients with preoperative therapy.
Pre-operative chemotherapy
Neoadjuvant or preoperative therapy has been evaluated largely in clinical T3 or node positive disease. Select controlled trials evaluating the use of preoperative chemotherapy in esophageal cancer are listed in Table 1. Perioperative chemotherapy for esophageal adenocarcinoma and SCC failed to improve any outcome compared to surgery alone in US Intergroup Trial 113 (32). This study administered three pre- and two postoperative cycles of infusional 5-FU combined with cisplatin (CF). Low rates of R0 resection were reported with (63%) or without chemotherapy (59%), with high rates of local tumor recurrence (27–29%) after curative surgery, and 5-year overall survival of 20% with or without chemotherapy. A larger trial from the UK, Medical Research Council esophageal cancer trial (OEO2) of preoperative CF (33,34), reported positive results and established a standard of care for preoperative CF in esophageal cancer. However, this trial also reported relatively low rates of R0 resection with (60%) or without chemotherapy (54%), as well as high rates of local recurrence after curative surgery (31–32%). A modest 6% improvement in 5-year overall survival was reported at long-term follow-up (17% to 23%) (35). The Federation Nationale des Centres de Lutte Contre le Cancer/Federation Francophone de Cancerologie Digestive (FNCLCC/FFCD) French trial treated 224 patients with adenocarcinoma of the lower esophagus, GE junction, or stomach with pre- and postoperative CF (36). Improvements in R0 resection (13%) and survival (13%) were reported for preoperative chemotherapy. This trial also reported high rates of local tumor recurrence after surgery (29–36%).
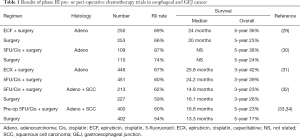
Full table
The Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial of perioperative chemotherapy in gastroesophageal cancer in the UK reported a survival benefit for perioperative chemotherapy (29). Patients with clinical stage II or higher adenocarcinomas of the lower third of the esophagus, GEJ, or stomach were treated, and the majority of the 503 patients treated (74%) had gastric primaries. Surgery alone was compared to 3 cycles of epirubicin, CF, and infusional 5-FU (ECF) pre- and 3 cycles post-surgery. Tumor down staging was modest with chemotherapy, with more T1–2 tumors in the chemotherapy group compared to surgery alone (52% vs. 37%) and more N0–1 tumors (84% vs. 72%). Similar numbers of patients had N0 disease with or without chemotherapy (31–27%). Progression free and overall survival were superior for the chemotherapy arm, with a 5-year overall survival increased from 23% to 36% with chemotherapy. Based on this trial, perioperative ECF became a new care standard in Western Europe and the US in esophagogastric cancer.
Concern about the adequacy of preoperative chemotherapy alone in esophageal and GEJ cancer patients continues given persistently lower rates of R0 resection for esophageal and GEJ cancers reported in contemporary trials. Two recent trials from the UK treated 1,900 patients with either preoperative CF or ECF, including 1,600 patients with esophageal and GEJ adenocarcinoma (30,31). Relatively low rates of R0 resection of 57–67% were reported for esophageal and Siewert I GEJ cancers, compared to 87% for more distal gastric cancers, on these studies using preoperative chemotherapy. These results have continued the debate about the potential need to include preoperative radiation therapy to ensure RO resection for patients with tumors involving the esophagus and GEJ.
Results of the FLOT4 trial, which compared treatment of GE junction and gastric cancer with perioperative ECF to a regimen combining docetaxel with infusional 5-FU and oxaliplatin (FLOT) in over 700 patients, were recently reported in abstract form (37). FLOT resulted in superior rates of R0 resection compared to ECF (77% to 84%), and improved progression free survival (18 to 30 months), and overall survival (35 to 50 months) with an estimated improvement in 5-year overall survival from 36% to 45%. FLOT may emerge as a new standard of care in the preoperative chemotherapy management of esophagogastric adenocarcinoma.
Post-operative chemotherapy
In East Asia, trials in resected gastric cancer have administered post-operative chemotherapy. However, fewer than 10% of tumors occur in the proximal stomach/GEJ, and it is unclear whether or not these results are applicable to Western patients with esophageal and GEJ cancers. Two trials, treating 2,000 patients with gastric cancer after D2 resection reported 9–10% improvements in 5-year survival for treatment with either 6 months of capecitabine plus oxaliplatin, or 1 year of S-1 (38,39).
Preoperative chemoradiotherapy
The landmark Radiation Therapy Oncology Group (RTOG) Trial 8501 combined 50 Gy of radiation therapy with concurrent CF for 2 cycles during and 2 cycles after radiation therapy, compared to radiation therapy alone (40). Long-term survival was achieved with chemoradiotherapy without surgery. No patients treated with radiation therapy alone survived beyond 3 years. At long-term follow-up, 26% of patients treated with combined chemoradiotherapy achieved 5-year survival, with the majority of patients treated on this trial having squamous cancers but adenocarcinoma patients also achieving a lesser but still measurable long-term survival (41). High rates of either local recurrence or persistence of disease after chemoradiotherapy were reported (52%), and the need for surgery after chemoradiotherapy has been continually debated.
Preoperative chemoradiation followed by surgery has been evaluated extensively in esophageal cancer. Selected randomized trials are outlined in Table 2. Largely underpowered early trials comparing pre-operative chemoradiation followed by surgery to surgery alone, had mixed results. Chemotherapy and radiotherapy followed by surgery for esophageal and GEJ cancers emerged as standard of care based on results from the Chemoradiotherapy for Oesophageal Cancer followed by Surgery Study (CROSS) (47,48). CROSS compared surgery alone to preoperative weekly carboplatin, paclitaxel, and 41.4 Gy of radiotherapy followed by surgery in esophageal and GEJ adenocarcinoma and squamous cell carcinoma. The majority of patients treated on trial had adenocarcinoma. In addition to increasing 5-year survival from 33% to 47% with combined preoperative chemoradiotherapy, higher rates of R0 resection (92% versus 69%) and relatively low rates of local recurrence (14% versus 34%) were reported with preoperative therapy compared to surgery alone. A pathologic complete response rate of 23% for adenocarcinoma was reported. The inclusion of squamous cancer patients makes interpretation of the results less clear: overall survival for adenocarcinoma was improved from 27.1 to 43.2 months (HR 0.73) with a more robust impact for squamous cancer (27.1 to 81.6 months, HR 0.48). A survival benefit was suggested more for clinical node negative patients (HR 0.50) compared to clinical node positive patients (HR 0.81). The short duration of chemotherapy administered during this treatment, 5 weeks of carboplatin and paclitaxel, has been criticized as inadequate systemic therapy.
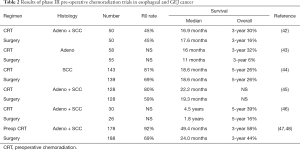
Full table
Preoperative chemoradiotherapy added to surgery in earlier clinical stage T1-2Nany or clinical T3N0 cancers have not clearly improved survival compared to surgery alone, including one recent trial from France (21). The majority of patients on this trial (72%) had squamous cancers. The high 93% R0 resection rate in the surgery-alone arm was not improved with pre-operative chemoradiation. Postoperative mortality was significantly increased in the chemoradiation arm (11.1% vs. 3.4%).
Additional preoperative chemotherapy cycles beyond 2 cycles of CF chemotherapy (30), the addition of epirubicin to FP (30), or the addition of induction chemotherapy cycles prior to chemoradiotherapy (35) all have failed to improve survival, arguing that the benefit from preoperative chemotherapy may be achieved with relatively brief chemotherapy exposure. The addition of targeted agents to chemoradiotherapy, including two trials adding the EGFR-targeted agent cetuximab to chemoradiotherapy (49,50), failed to improve any outcome. A pilot trial combining the vascular endothelial growth factor (VEGF) targeted agent bevacizumab with chemoradiotherapy (51), and a randomized trial adding bevacizumab to preoperative chemotherapy (31) also failed to improve outcome. RTOG trial 1010 (NCT01196390) recently completed accrual and evaluated the addition of trastuzumab to preoperative carboplatin, paclitaxel, and radiation therapy in HER2 positive esophageal and GEJ cancers, and results are expected within the next 1–2 years.
An ongoing trial in GEJ and gastric cancer, TOPGEAR, is comparing the use of perioperative chemotherapy with ECF with or without preoperative radiotherapy (NCT 01924819). Other trials evaluating preoperative chemotherapy with or without radiotherapy in esophageal and GEJ cancers include a German trial comparing preoperative 5-FU, oxaliplatin, and docetaxel vs. the CROSS approach (NCT02509286), and an Irish study comparing preoperative chemotherapy with ECF to CROSS (NCT01726452).
A trial attempting to compare pre-operative chemoradiation to pre-operative chemotherapy was reported by the German POET trial (PreOperative Chemotherapy or Radiochemotherapy in Esophagogastric Adenocarcinoma Trial). Patients with GEJ adenocarcinomas were randomized to either 5-FU/leucovorin/CF followed by surgery or 5-FU/leucovorin/CF followed by chemoradiation with CF/etoposide and then surgery (52). The trial closed due to poor accrual at only 118 patients and was underpowered to achieve any definitive conclusion. Patients treated with chemoradiation had a higher pathologic complete response rate (15.6% vs. 2%) and node negative status at surgery (64.4% vs. 36.7%) compared to treatment with chemotherapy alone. There were trends toward improves local control (76.5% vs. 59%) and 3-year overall survival (47.4% vs. 27.7%) for the chemoradiation group which did not reach statistical significance.
A similar non-significant trend toward improved outcomes with pre-operative chemoradiation over chemotherapy was also suggested in the meta-analysis reported by Sjoquist et al., which revealed an all-cause mortality HR of 0.88 (95% CI: 0.76–1.01, P=0.07) favoring chemoradiation (53).
Post-operative chemoradiation
In the US, one additional alternative is post-operative chemoradiation for GEJ and gastric cancers undergoing up front resection, based on the results of the Intergroup 116 trial (54,55). This trial randomized 556 patients (20% of whom had tumors that involved the GEJ) to adjuvant chemotherapy and chemoradiation with bolus 5-FU/leucovorin or to observation alone following surgery. Overall survival was improved by 10% favoring postoperative therapy. This trial had relatively poor surgical quality, with 54% of patients having less than a D1 or D2 resection. Because many patients after esophagogastrectomy fail to recover to the point of tolerating post-operative therapy, and given the potential downstaging achieved by preoperative therapy, postoperative chemoradiotherapy for esophageal or GEJ cancers is rarely applied.
Salvage surgery after neoadjuvant chemoradiotherapy
For patients with a poor performance status or with advanced age and medical comorbidities, given a potentially high risk for either mortality or severe complications from esophagectomy, definitive chemoradiotherapy without surgical resection is considered (56,57). Local recurrence rates after definitive chemoradiotherapy are high and are observed in 40–75% of patients (58-60). Patients with local regional recurrence after definitive chemoradiotherapy may be considered for salvage surgery (56). After salvage surgery, a higher mortality rate is reported as well as increased rates of anastomotic leak and pulmonary complications (56).
Targeted agents, genomic analysis, and PET scan imaging
Targeted therapy trials have largely focused on the VEGF pathway, and receptor associated tyrosine kinase pathways such as EGFR, HER2, and MET. In metastatic disease, response and survival benefits have been observed in phase III trials of the agent ramucirumab targeting the VEGFR2 receptor, and trastuzumab targeting the HER2 receptor (61-63). As noted above, trials combining the EGFR inhibitors cetuximab or the VEGF inhibitor Bevacizumab have failed to improved outcome when added to either chemoradiotherapy or preoperative chemotherapy (31,49,50). Results from a trial combining trastuzumab with preoperative chemoradiotherapy in HER2 positive esophageal and GEJ cancer are pending.
Genomic analyses of gastroesophageal cancers using broad genomic screening for gene mutation, amplification, and deletions have recently been reported. Remarkably similar genomic profiles have emerged from genomic studies evaluating Western and Asian patients with gastric cancer (64,65). Molecular subgroups identified in US studies under The Cancer Genome Atlas (TCGA) project, include (I) genomically unstable tumors with higher rates of receptor associated tyrosine kinase pathway gene amplification (HER2, EGFR, MET, FGFR), high rates of p53 mutation, and amplification of VEGFA and cell cycle pathways; (II) genomically stable tumors with relatively few mutations and presence of CDH-1 and RHO-A mutation; (III) tumors with higher rates of gene hypermethylation leading to a higher mutation burden with high rates of microsatellite instability (MSI); and (IV) tumors associated with EBV infection and amplification of potential immune related pathways including over expression of PDL-1 and PDL-2 ligands. Correlating anatomic location and conventional histology, the vast majority of esophageal and GEJ adenocarcinomas fall under the genomically unstable category and 50% of distal gastric cancers fall into this category (66). Therapeutic implications of these subgroups include studying receptor associated kinase agents in genomically unstable tumors, and immune checkpoint inhibitors in MSI or EBV associated tumors given the higher mutational burden and higher expression of PDL-1 and PDL-2 ligands in these subgroups. A recent genomic analysis of 129 esophageal and GEJ adenocarcinomas identified three targetable subgroups, including enrichment for a BRCA like signature, tumors with a higher potential mutational burden, and tumors with a mutational pattern consistent with an aging imprint, with potential therapeutic implications including use of DNA damaging agents and immune checkpoint inhibitors (67). Stratification of patients on the basis of molecular profile will be obligatory in future trials given the potential for intrinsic biologic differences in these subsets.
Agents targeting immune checkpoint pathways mediated by the CTLA-4 and PD-1 pathways have also undergone recent extensive evaluation. Recent phase II and phase III trials indicate a small but consistent signal of activity for anti PD-1 or PDL-1 agents in esophageal and GEJ cancers, with response rates ranging from 10–20% with a suggestion of higher response rates in patients with overexpression of PDL-1 (68,69). A recent phase III trial in gastric and GEJ cancer patients refractory to chemotherapy compared supportive care to treatment with the anti PD-1 agent nivolumab (69). Overall survival as well as response rate and progression free survival were improved for patients treated with nivolumab compared to placebo. These agents will likely be incorporated into clinical trials of neoadjuvant or adjuvant chemotherapy and radiation therapy.
Imaging during induction chemotherapy with PET scan may identify patients with early PET response who appear to have better clinical outcomes than PET non-responders, with higher rates of pathologic response, R0 resection, and potentially improved survival (70,71). A preliminary report of Cancer and Acute Leukemia Group B (CALGB)/ Alliance trial 80803 indicated that use of PET scan to direct chemotherapy during preoperative chemoradiotherapy may enhance the rate of pathologic complete response (72). Patients were randomized to induction chemotherapy with either weekly carboplatin and paclitaxel or FOLFOX, and a PET scan was performed after 5–6 weeks of therapy. PET non-responders changed chemotherapy during radiation therapy to the alternative regimen, i.e., PET non-responders to carboplatin and paclitaxel were changed to infusional 5-FU and oxaliplatin during radiotherapy, and FOLFOX non-responders were changed to carboplatin and paclitaxel during radiotherapy. Pathologic complete responses in the cross over patients were observed in 17–19%, suggesting an improvement over an expected pathologic complete response of 5% from historical controls. Survival data from this trial are still pending.
Conclusions
Preoperative therapy with chemotherapy or chemoradiotherapy improves outcome over surgery alone and the debate about the role of radiation therapy with preoperative chemotherapy continues. The identification of agents targeting HER2, the VEGF pathway, and immune checkpoints has led to completed, ongoing, or planned clinical trials adding these agents to preoperative therapy. Genomic profiling has identified unique molecular subsets of these cancers, which may help guide new drug development and impact on the design of the next generation of neoadjuvant trials. The role of PET scan imaging to guide preoperative therapy continues to be studied in clinical trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: Consulting work with Amgen, Bayer, Astra-Zeneca, Merck, Roche, Bristol Myers Squibb, Pieris, Lilly.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol 2004;31:450-64. [Crossref] [PubMed]
- Devesa SS, Fraumeni JF Jr. The rising incidence of gastric cardia cancer. J Natl Cancer Inst 1999;91:747-9. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global Cancer Statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Haverkamp L, Seesing MF, Ruurda JP, et al. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus 2017;30:1-7. [PubMed]
- Reavis KM, Smith BR, Hinojosa MW, et al. Outcomes of esophagectomy at academic centers: an association between volume and outcome. Am Surg 2008;74:939-43. [PubMed]
- Coupland VH, Lagergren J, Lüchtenborg M, et al. Hospital volume, proportion resected and mortality from oesophageal and gastric cancer: a population-based study in England, 2004-2008. Gut 2013;62:961-6. [Crossref] [PubMed]
- Visbal AL, Allen MS, Miller DL, et al. Ivor Lewis esophagogastrectomy for esophageal cancer Ann Thorac Surg 2001;71:1803-8. [Crossref] [PubMed]
- McKeown KC. Total three-stage oesophagectomy for cancer of the oesophagus Br. J. Surg 1976;63:259-62. [Crossref] [PubMed]
- Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg 1978;76:643-54. [PubMed]
- Orringer MB. Transhiatal esophagectomy without thoracotomy for carcinoma of the thoracic esophagus. Ann Surg 1984;200:282-8. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000. [Crossref] [PubMed]
- Wei MT, Zhang YC, Deng XB, et al. Transthoracic vs transhiatal surgery for cancer of the esophagogastric junction: a meta-analysis. World J Gastroenterol 2014;20:10183-92. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Zhou C, Zhang L, Wang H, et al. Superiority of Minimally Invasive Oesophagectomy in Reducing In-Hospital Mortality of Patients with Resectable Oesophageal Cancer: A Meta-Analysis. PLoS One 2015;10:e0132889. [Crossref] [PubMed]
- Ruurda JP, van der Sluis PC, van der Horst S, et al. Robot-assisted minimally invasive esophagectomy for esophageal cancer: A systematic review. J Surg Oncol 2015;112:257-65. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, Verhage RJ, et al. Oncologic Long-Term Results of Robot-Assisted Minimally Invasive Thoraco-Laparoscopic Esophagectomy with Two-Field Lymphadenectomy for Esophageal Cancer. Ann Surg Oncol 2015;22 Suppl 3:S1350-6. [Crossref] [PubMed]
- Markar SR, Gronnier C, Pasquer A, et al. French Eso-Gastric Tumors (FREGAT) working group – Federation de Recherche EN CHirurgie (FRENCH) – Association Francaise de Chirurgie (AFC). Role of neoadjuvant treatment in clinical T2N0M0 oesophageal cancer: results from a retrospective multi-center European study. Eur J Cancer 2016;56:59-68. [Crossref] [PubMed]
- Speicher PJ, Ganapathi AM, Englum BR, et al. Induction therapy does not improve survival for clinical stage T2N0 esophageal cancer. J Thorac Oncol 2014;9:1195-201. [Crossref] [PubMed]
- Mariette C, Dahan L, Mornex F, et al. Surgery Alone Versus Chemoradiotherapy Followed by Surgery for Stage I and II Esophageal Cancer: Final Analysis of Randomized Controlled Phase III Trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [Crossref] [PubMed]
- Dolan JP, Kaur T, Diggs BS, et al. Significant understaging is seen in clinically staged T2N0 esophageal cancer patients undergoing esophagectomy. Dis Esophagus 2016;29:320-5. [Crossref] [PubMed]
- Hardacker TJ, Ceppa D, Okereke I, et al. Treatment of clinical T2N0M0 esophageal cancer. Ann Surg Oncol 2014;21:3739-43. [Crossref] [PubMed]
- Crabtree TD, Yacoub WN, Puri V, et al. Endoscopic ultrasound for early stage esophageal adenocarcinoma: implications for staging and survival. Ann Thorac Surg 2011;91:1509-15. [Crossref] [PubMed]
- Zhang JQ, Hooker CM, Brock MV, et al. Neoadjuvant chemoradiation therapy is beneficial for clinical stage T2 N0 esophageal cancer patients due to inaccurate preoperative staging. Ann Thorac Surg 2012;93:429-35. [Crossref] [PubMed]
- Samson P, Puri V, Robinson C, et al. Clinical T2N0 Esophageal Cancer: Identifying Pretreatment Characteristics Associated With Pathologic Upstaging and the Potential Role for Induction Therapy. Ann Thorac Surg 2016;101:2102-11. [Crossref] [PubMed]
- Chen WH, Chao YK, Chang HK, et al. Long-term outcomes following neoadjuvant chemoradiotherapy in patients with clinical T2N0 esophageal squamous cell carcinoma. Dis Esophagus 2012;25:250-5. [Crossref] [PubMed]
- Martin JT, Worni M, Zwischenberger JB, et al. The role of radiation therapy in resected T2 N0 esophageal cancer: a population-based analysis. Ann Thorac Surg 2013;95:453-8. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Alderson D, Langley RE, Nankivell MG, et al. Neoadjuvant chemotherapy for resectable oesophageal and junctional adenocarcinoma: Results from the UK Medical Research Council randomised OEO5 trial (ISRCTN 01852072). J Clin Oncol 2015;33 suppl:abstr 4002.
- Cunningham D, Stenning SP, Smyth EC, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol 2017;18:357-70. [Crossref] [PubMed]
- Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339:1979-84. [Crossref] [PubMed]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727-33. [Crossref] [PubMed]
- Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27:5062-7. [Crossref] [PubMed]
- Ajani JA, Xiao L, Roth JA, et al. A phase II randomized trial of induction chemotherapy versus no induction chemotherapy followed by preoperative chemoradiation in patients with esophageal cancer. Ann Oncol 2013;24:2844-9. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal adenocarcinoma: an FNLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Al-Batran SE, HomannN, Schmalenberg H, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. J Clin Oncol 2017;35 suppl:abstr 4004.
- Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387-93. [Crossref] [PubMed]
- Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:1389-96. [Crossref] [PubMed]
- Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593-8. [Crossref] [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305-13. [Crossref] [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [Crossref] [PubMed]
- Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997;337:161-7. [Crossref] [PubMed]
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. [Crossref] [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiation for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Shapiro J, van Lanschot JB, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Crosby T, Hurt CN, Falk S, et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol 2013;14:627-37. [Crossref] [PubMed]
- Ilson DH, Moughan J, Suntharalingam M, et al. RTOG 0436: A phase III trial evaluating the addition of cetuximab to paclitaxel, cisplatin, and radiation for patients with esophageal cancer treated without surgery. J Clin Oncol 2014;32 suppl:abstr 4007.
- Bendell JC, Meluch A, Peyton J, et al. A phase II trial of preoperative concurrent chemotherapy/radiation therapy plus bevacizumab/erlotinib in the treatment of localized esophageal cancer. Clin Adv Hematol Oncol 2012;10:430-7. [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. NEJM 2001;345:725-30. [Crossref] [PubMed]
- Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed Intergroup Study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012;30:2327-33. [Crossref] [PubMed]
- Markar SR, Karthikesalingam A, Penna M, et al. Assessment of short-term clinical outcomes following salvage esophagectomy for the treatment of esophageal malignancy: Systematic review and pooled analysis. Ann Surg Oncol 2014;21:922-31. [Crossref] [PubMed]
- Wakui R, Yamashita H, Okuma K, et al. Esophageal cancer: definitive chemoradiotherapy for elderly patients. Dis Esophagus 2010;23:572-9. [Crossref] [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [Crossref] [PubMed]
- Kranzfelder M, Schuster T, Geinitz H, et al. Meta-analysis of neoadjuvant treatment modalities and definitive non-surgical therapy for oesophageal squamous cell cancer. Br J Surg 2011;98:768-83. [Crossref] [PubMed]
- Takeuchi S, Ohtsu A, Doi T, et al. A retrospective study of definitive chemoradiotherapy for elderly patients with esophageal cancer. Am J Clin Oncol 2007;30:607-11. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Deng N, Doh LK, Wang H, et al. A comprehensive surgery of genomic alterations in gastric cancer reveals systemic patterns molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 2012;61:673-84. [Crossref] [PubMed]
- The Cancer Genome Atlas Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169-75. [Crossref] [PubMed]
- Secrier M, Li X, de Silva N, et al. Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat Genet 2016;48:1131-41. [Crossref] [PubMed]
- Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717-26. [Crossref] [PubMed]
- Kang YK, Satoh T, Ryu MH, et al. Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): A double-blinded, randomized, phase III trial. J Clin Oncol 2017;35 suppl 4S;abstract 2.
- Ott K, Weber WA, Lordick F, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol 2006;24:4692-8. [Crossref] [PubMed]
- Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 2007;8:797-805. [Crossref] [PubMed]
- Goodman KA, Niedzwiecki D, Hall N, et al. Initial results of CALGB 80803 (Alliance): A randomized phase II trial of PET scan-directed combined modality therapy for esophageal cancer. J Clin Oncol 2017;35 suppl 4S:abstract 1.


