Systemic therapy for esophagogastric cancer: targeted therapies
Background
In the United States, cancers of the esophagus are uncommon but aggressive. In 2017, an estimated 16,940 patients will be diagnosed, with an estimated 15,690 deaths from this disease (1). These poor outcomes notwithstanding, survival has actually improved over time. In the period from 1975 to 1977 and 2000 to 2007, 5-year survival for esophageal cancer has increased from 5% to 19%. This likely reflects improved outcomes with primary surgical management and the now accepted and standard usage of neoadjuvant chemotherapy or chemoradiation combined with surgery. In the palliative setting, improvements in chemotherapy have largely been incremental.
Scientific rationale for targeted therapies
Most investigators believe that the potential for making significant progress lies in understanding and exploiting the molecular biology of these tumors. The focus of recent study has shifted toward testing newer agents that target specific molecular abnormalities known to occur in esophagogastric cancers (EGCs).
Gene expression profiling of 296 EGCs from a Western population by Dulak et al. found amplified genes in 37% of samples, including in several of the therapeutically targetable kinases that are discussed here [specifically epidermal growth factor receptor (EGFR), Her2 and MET], along with fibroblast growth factor receptor and K-ras (2). A similar analysis of 193 tumors (mostly from Singapore and East Asia) also identified amplifications of the exact same five targets (3). On the other hand, mutations in the K-ras and B-raf oncogenes, which are common in some other solid tumors, are rare in EGCs (4).
Therefore, the molecular targets of agents that are currently under active clinical evaluation include Her2, vascular endothelial growth factor (VEGF) or its receptor, MET and its ligand, hepatocyte growth factor (HGF), mammalian target of rapamycin (mTOR) and poly (ADP-ribose) polymerase (PARP) and claudin 18.2 (CLDN18.2) (Figure 1).
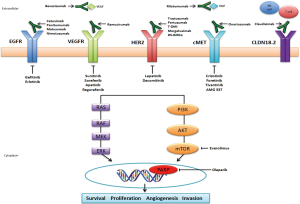
EGFR
EGFR or ERBB1 is a member of the ERBB transmembrane growth factor receptor family, which initiates signal transduction by activation of a receptor-associated tyrosine kinase (TK); ERBB also includes ERBB2 (Her2), ERBB3 and ERBB4 (5). Downstream pathways activated by signaling through this family include the MAP kinase pathway and the PI3K/Akt pathway.
In EGCs, EGFR overexpression by immunohistochemistry (IHC) or gene amplification by fluorescent in situ hybridization (FISH) occur in 30–90% of tumors and correlate with increased invasion, a more advanced stage, and a more poorly differentiated histology and a worse prognosis (6-9).
Anti-EGFR monoclonal antibodies (cetuximab and panitumumab)
Four completed phase III trials of cetuximab and panitumumab have been reported or published. Individually, each of these studies has been negative; collectively, they have dampened enthusiasm for further evaluation of these drugs in an unselected population.
In the metastatic setting, two studies have been published. The REAL3 study randomized 553 patients with advanced EG adenocarcinoma to EOC (epirubicin/oxaliplatin/capecitabine) chemotherapy alone or the addition of panitumumab (10). Unfortunately, the addition of panitumumab resulted in a statistically significant inferior OS [11.3 vs. 8.8 months, hazard ratio (HR) =1.37, P=0.013], which was the primary end-point. The addition of panitumumab also resulted in a trend toward inferior progression-free survival (PFS) (6.0 vs. 7.4 months, HR =1.22, P=0.068) and did not improve RRs (46% vs. 42%, P=0.42). Toxicities were also increased with adding panitumumab and included higher rates of grade 3/4 diarrhea, mucositis, rash and hypomagnesemia, all class-effects of anti-EGFR mAbs.
These disappointing results cannot be easily explained. One possibility is that increased toxicities seen when panitumumab was initially added to full-dose EOC chemotherapy led to dose reductions in oxaliplatin (by 23%) and capecitabine (by 20%). These dose reductions in chemotherapy—coupled with a lack of biologic benefit or even harm from the addition of panitumumab—could have resulted in the inferior OS in the experimental arm.
Similarly, EXPAND, a phase III trial of 904 patients with advanced EG adenocarcinoma treated with capecitabine/cisplatin with or without cetuximab, also failed to show a benefit in PFS, the primary end-point (4.4 vs. 5.6 months, HR =1.09, P=0.32) (11). RRs (30% vs. 29%, P=0.77) and OS (9.4 vs. 10.7 months, P=0.95) were also not improved. There was no difference in the primary outcome based on EGFR expression by IHC. Again, toxicities were increased in the cetuximab arm, including grade 3/4 diarrhea, rash, hand-foot syndrome and hypomagnesemia.
While all of these studies have focused on adenocarcinoma histology, a German randomized phase II study evaluated 5-FU/cisplatin with or without cetuximab in 62 esophageal SCC patients (12). The RR rate was not improved with the addition of cetuximab (19% vs. 13%, P=0.79) and there was only a trend toward improved time-to-treatment-failure (3.4 vs. 1.6 months, P=0.25) and OS (9.5 vs. 5.5 months, P=0.32). Despite these equivocal results, a phase III study was initiated in Germany to randomize patients to 5-FU/cisplatin with or without panitumumab. The study was closed after an interim analysis demonstrated futility and results just presented in abstract form show no improvement in survival and increased toxicities for adding panitumumab (13).
In the locally advanced setting, two studies have evaluated the addition of cetuximab to chemoradiation for locally advanced esophageal and GE junction (GEJ) tumors. The U.K. SCOPE-1 study treated 258 patients with capecitabine/cisplatin and radiation with or without cetuximab (14). Seventy-three percent of patients had SCC histology and 60% had stage III disease. The study was stopped after a pre-planned analysis of the first 180 patients who had completed 24 weeks of follow-up occurred because it met pre-determined criteria for futility. The primary endpoint of being treatment-failure free at 24 weeks was lower in the cetuximab-arm [66.4%, 90% confidence interval (CI): 58.6–73.6] vs. the standard arm (76.9%, 90% CI: 69.7–83.0). The patients who received cetuximab also had inferior OS (22.1 vs. 25.4 months, HR =1.45, P=0.035).
Finally and most recently, the results of the Radiation Therapy Oncology Group (RTOG) 0436 study were presented in abstract form (15). This study evaluated cisplatin/paclitaxel and radiation with or without cetuximab in the non-operative setting for locally advanced esophageal SCC and adenocarcinoma. There was no difference in outcomes between both arms, confirming a lack of benefit for cetuximab combined with chemoradiation.
Other anti-EGFR mAbs
Matuzumab is another humanized anti-EGFR mAb. In a randomized phase II study, 72 patients with advanced EG adenocarcinoma received ECX chemotherapy (epirubicin/cisplatin/capecitabine) with or without matuzumab (16). Its addition did not improve RRs or survival outcomes.
Nimotuzumab is also a humanized anti-EGFR mAb. A phase II study investigating the combination of nimotuzumab and irinotecan was negative (17). A Japanese/Korean phase III study is currently accruing 400 patients with GEJ/gastric adenocarcinoma to second-line irinotecan with or without nimotuzumab (NCT01813253) but is unlikely to complete accrual given the negative phase II results of this combination.
Anti-EGFR tyrosine kinase inhibitors (TKIs)
TKIs are oral agents which lead to complete inhibition of EGFR autophosphorylation and signal transduction (18). Two oral TKIs, gefitinib and erlotinib, have been investigated in advanced EGCs, with negative outcomes. In addition to a number of mixed phase II studies (19,20), the phase III COG (Cancer Oesophagus Gefitinib) trial enrolled 450 patients, who had received ≤2 prior regimens and were randomized to gefitinib vs. placebo (21). There was a clinically insignificant increase in PFS (49 vs. 35 days, HR =0.795, P=0.017), with no improvement in overall survival.
Phase II studies of erlotinib have also not suggested activity. A phase II study of second-line erlotinib was performed here at Memorial Sloan-Kettering Cancer Center (MSKCC) (22). In this study of 30 patients with advanced esophageal and GEJ tumors, 2 (7%) had PRs while 10 (33%) had SD. Both patients with a PR had SCC histology (2 of 13 patients vs. 0 of 17 patients with adenocarcinoma), EGFR overexpression and nodal-limited disease. Median TTP was longer in SCC vs. adenocarcinoma patients (3.3 vs. 1.6 months, P=0.026).
The Southwest Oncology Group (SWOG) performed a phase II study of erlotinib as first-line therapy for gastric/GEJ adenocarcinoma. The patients were stratified into a GEJ (44 patients) and gastric (26 patients) stratum (23). There were no responses in the patients with gastric primaries. In the GEJ stratum, the RR was 9% (1 complete response or CR and 3 PRs). The median OS was 3.5 and 6.7 months for the gastric and GEJ strata respectively.
It is clear from these studies evaluating both erlotinib and gefitinib, they do not have much activity as single-agents in EGCs.
Anti-HER2 therapy
Her2 is another member of the ERBB TK receptor family. Ligand binding to these receptors leads to dimerization of a receptor either with itself or another member of the ERBB family. At least nine such homo- and heterodimers exist. In this network, Her2 plays a major coordinating role since each receptor with a specific ligand appears to prefer Her2 as its heterodimeric partner. This preference is further biased by overexpression of Her2, as seen in many types of human cancer cells (24).
In EGCs, Her2 over-expression has been variably demonstrated in esophageal SCC (mean 23%, range, 0–52%) and GEJ adenocarcinoma (mean 22%, range, 0–43%) (25,26). The wide range of expression reflects the differences in receptor testing based on IHC or FISH, as well as the varied cancer stages of patients.
The significance of Her2 expression as a prognostic/predictive marker is unclear. In esophageal SCC, Her2 over-expression has been correlated with extramural invasion, poor response to neoadjuvant chemotherapy and inferior 5-year survival (27). However, a more recent study from the Mayo Clinic suggested that Her2 over-expression is actually associated with a lower tumor grade, fewer malignant lymph nodes and the presence of Barrett’s esophagus (28). In gastric and GEJ adenocarcinoma, some studies have demonstrated a correlation between Her2 amplification as determined by FISH with increasing depth of invasion, lymph node and distant organ metastasis and overall poor survival (29). The worse prognosis conveyed by Her2 positivity appears to be confirmed by a meta-analysis of 49 studies involving 11,337 patients with localized and metastatic tumors (30).
However, more recent studies not included in this meta-analysis have suggested otherwise. A study by our group indicates that Her2 over-expression is more common in intestinal vs. diffuse/mixed histology (33% vs. 8%, P=0.001) but that it is not an independent prognostic factor in the metastatic setting (31). Similarly, patients with Her2 positive tumors actually had improved RRs and OS in either arm of the EXPAND trial compared to patients with Her2 negative tumors (11).
In the locally advanced setting, large retrospective reviews also did not show Her2 to be prognostic or predictive either of benefit from peri-operative chemotherapy (in the U.K. MAGIC study with epirubicin/cisplatin/5-FU) (32) or from adjuvant chemotherapy following up-front surgery (in the Japanese ACTS-GC trial with the oral 5-FU pro-drug S-1) (33).
Part of the discrepancy of these results may arise because of differences in IHC staining and scoring for Her2. The more contemporary studies cited here used IHC and FISH techniques similar to those used in the ToGA study (discussed below), which may make their conclusions more relevant.
Trastuzumab
Trastuzumab, a mAb against Her2, was the first targeted therapy approved by the U.S. Food and Drug Administration (FDA) for EGCs. In the pivotal ToGA (Trastuzumab for Gastric Cancer) trial, the addition of trastuzumab to a fluoropyrimidine/cisplatin doublet for patients with GEJ and gastric adenocarcinomas, whose tumors were Her2 positive by IHC (3+) or FISH (Her2/CEP17 ratio ≥2), improved outcomes (34). RRs (47% vs. 35%, P=0.0017), median PFS (6.7 vs. 5.5 months, P=0.0002) and OS (13.8 vs. 11.1 months, P=0.0046) were all improved with the addition of trastuzumab. The greatest benefit seen for the addition of trastuzumab was in high Her2 over-expressors with IHC 3+ or FISH-positive/IHC 2+ patients. Based on this differential benefit, trastuzumab is approved in the European Union only for this subgroup of high Her2 over-expressors; in the U.S., it is approved for any patient who met the eligibility criteria for the ToGA study, including patients with FISH-positive status only.
The phase III trial Heloise study, which was recently published, compared standard-of-care trastuzumab (8 mg/kg loading followed by 6 mg/kg every 3 weeks) plus chemotherapy (cisplatin/capecitabine) with higher-dose (HD) trastuzumab (8 mg/kg loading followed by 10 mg/kg every 3 weeks) plus chemotherapy in first-line HER2 positive metastatic gastric or GEJ adenocarcinoma (35). HD trastuzumab arm did not increase efficacy and there was no difference in OS (12.5 months in the standard of care trastuzumab arm vs. 10.6 months in the HD trastuzumab arm, HR =1.24; 95% CI: 0.86−1.78; P=0.2401).
Trastuzumab is currently undergoing evaluation in the peri-operative setting. Based on the results of a small phase I study, the RTOG 1010 study (NCT01196390) is randomizing patients with Her2-positive esophageal and GEJ adenocarcinomas to pre-operative chemoradiation with carboplatin/paclitaxel with or without trastuzumab. Patients in the trastuzumab arm also receive adjuvant trastuzumab for one year following surgery. In addition, a German group has completed accrual to the single-arm phase II HerFLOT study (NCT01472029), which is evaluating the pathologic complete response rate of pre-operative 5-FU/leucovorin/oxaliplatin/docetaxel and trastuzumab; patients on this study also receive adjuvant trastuzumab.
Other anti-HER mAbs
Building on the results of the ToGA study and mirroring strategies and drugs that have proven to be beneficial in Her2-positive breast cancer, other anti-Her2 antibody strategies are currently undergoing phase III evaluation. Trastuzumab emtansine (T-DM1) is an antibody-drug conjugate of trastuzumab and DM1 (a microtubule inhibitor derived from maytansine), which has been approved by the FDA for use in Her2-positive breast cancer that is refractory to trastuzumab and a taxane, based on the phase III EMILIA study (36). T-DM1 binds to Her2, releasing DM1 into the cytoplasm via receptor-mediated internalization and resulting in apoptosis. The randomized phase II/III GATSBY trial compared T-DM1 vs. docetaxel/paclitaxel in the second-line treatment of Her2-positive metastatic gastric cancer progressing on trastuzumab based therapy (37). Results revealed no improvement in OS (7.9 vs. 8.6 months, P=0.86) or PFS (2.7 vs. 2.9 months, P=0.31) for patients treated with trastuzumab emtansine compared with patients treated with a taxane and the proportion of patients who achieved an objective response was 20·6% vs. 19·6%.
Pertuzumab is a humanized monoclonal antibody that binds to the domain II dimerization arm of Her2, leading to inhibition of Her2 dimerization with other ErBB family members and antibody dependent cell-mediated cytotoxicity (38,39). Pertuzumab was initially FDA-approved in combination with trastuzumab and docetaxel for previously untreated Her2-positive breast cancer on the basis of the CLEOPATRA study (40).
The phase II JOSHUA study randomized 30 Her2-positive EG adenocarcinoma patients to two different doses of pertuzumab in combination with capecitabine/cisplatin and trastuzumab (41). Fifteen patients received pertuzumab at a dose of 840 mg every 3 weeks for 6 cycles and the other 15 patients received 840 mg of pertuzumab for cycle 1 followed by 420 mg every 3 weeks for the next 5 cycles. Both doses reached the pre-defined trough concentrations of pertuzumab on day 43. The disease-control rates (complete plus partial response plus stable disease) at the end of 6 cycles were 82% and 100% respectively in each arm. Toxicities appeared to be similar in both arms and diarrhea, mostly grade 1/2, was the most common toxicity. The higher dose of 840 mg every 3 weeks was selected for the ongoing phase III JACOB study (NCT01774786), which has completed accrual and is randomizing 780 patients to fluoropyrimidine/cisplatin and trastuzumab with or without pertuzumab.
Similar to trastuzumab, the incorporation of pertuzumab to a trastuzumab/chemotherapy backbone is being investigated in the perioperative setting. The PETRARCA trial is phase II/III study investigating FLOT vs. FLOT/trastuzumab/pertuzumab in the perioperative setting. Patients will receive 4 cycles pre- and post-surgery (NCT02581462) and INNOVATION trial is a phase II neoadjuvant study of cisplatin/5FU + trastuzumab ± pertuzumab in patients with Her2+ adenocarcinoma of gastric/GEJ origin (NCT02205047).
In addition to blocking Her2, trastuzumab mediates antibody-dependent cell-mediated cytotoxicity (ADCC) (42). Margetuximab is an anti-Her2 monoclonal antibody that binds with elevated affinity to both the lower and higher affinity forms of CD16A, an Fc-receptor important for ADCC against tumor cells. A phase I study investigated margetuximab in patients with Her2+ tumors, 66% of whom had been treated with at least 1 line of Her2-directed therapy (43). A total of 66 patients were enrolled, 20 of which had EGC. The greatest signal of activity was seen in breast cancer but 2 (10%) patients with EGC had a PR. Margetuximab has entered a phase III trial (SOPHIA) in Her2+ breast cancer (NCT02492711). It is also being investigated in EGC in a phase I study combined with pembrolizumab an anti-PD-1 mAb (NCT02689284).
Lastly, another antibody drug conjugate targeting Her2 has entered early phase I studies. DS-8201a is composed of a humanized IgG1 mAb identical to trastuzumab and a derivative of exatecan, which is a topoisomerase I inhibitor is used as the cytotoxic component. The drug-to-antibody ratio (DAR) for DS-8201a is 8, compared to a DAR of 3.5 for T-DM1 (44). This results in more drug being delivered into the Her2+ cancer cell. A phase I study has begun enrollment, testing this drug in both Her2+ EGC and breast cancer (NCT02564900).
Anti-HER2 TKI agents
Lapatinib is an oral TKI that has activity against EGFR and Her2. To date, results of two phase II evaluations of lapatinib have been reported. The SWOG evaluated lapatinib as first-line therapy in 47 unselected patients with advanced gastric cancer (45). Only 4 patients (9%) had a confirmed PR; the median time-to-treatment failure was 1.9 months and median OS was 4.8 months.
Despite the modest results, the randomized phase III TyTAN study evaluated the addition of lapatinib to second-line paclitaxel and did show some activity (46). Two hundred and sixty-one Asian patients with Her2 positive gastric cancer by FISH were enrolled. In the intention-to-treat population the median OS was not improved, however, a planned subset analysis of patients who were also 3+ by IHC revealed an OS benefit for lapatinib (14 vs. 7.6 months, P=0.0176). Patients on this study were essentially trastuzumab naive, with only 6% having received prior trastuzumab in the lapatinib arm vs. 5% in the paclitaxel alone arm.
Ultimately, the question of whether there is clear benefit for lapatinib was determined with the results of the phase III LOGiC trial (47). This study added lapatinib to first-line chemotherapy with capecitabine/oxaliplatin for Her2-overexpressing EGCs. This was a negative trial, with no improvement in OS, the primary endpoint (12.2 vs. 10.5 months; HR =0.91; 95% CI: 0.73−1.12; P=0.3492). However, the lapatinib arm showed a statistically significant higher response rate (53% vs. 39%; P=0.0031). Also in a preplanned exploratory subgroup analysis, Asian patients experienced a more pronounced OS benefit (16.5 vs. 10.9 months; HR =0.68; 95% CI: 0.48−0.96; P=0.0261).
Finally, a trial of a novel pan-ERBB irreversible inhibitor, dacomitinib, enrolled 27 Korean patients with advanced Her2-positive gastric cancer (48). This was a heavily pre-treated population and 41% of patients had received ≥3 prior chemotherapy regimens; 22% had received prior anti-Her2 therapy. The response rate was 7.4% and the disease control rate (PR plus SD) was 40.7%. Median PFS was 2.1 months and median OS was 7.1 months. Such survival data are promising in such a chemorefractory patient population, although gastric cancer patients who have received more than three prior chemotherapy regimens and remain sufficiently well to participate in a clinical trial are clearly not representative of the general treatment population.
VEGF
Angiogenesis and its targeting with VEGF-directed therapy is being investigated in many solid tumor malignancies. Folkman and others have provided compelling evidence linking tumor growth and metastases with angiogenesis (49).
In esophageal cancer, VEGF is over-expressed in 30–60% of patients, with several studies demonstrating a correlation between high levels of VEGF expression, advanced stage and poor survival in patients undergoing esophagectomy (50-54). Similarly, increased VEGF expression on tumors and increased serum VEGF levels have been correlated with worse prognoses in gastric cancer (55,56).
Anti-VEGF mAb (bevacizumab)
The phase III AVAGAST trial evaluated the addition of bevacizumab, a monoclonal antibody against VEGF, to capecitabine/cisplatin, as first-line therapy for patients with advanced EG adenocarcinoma (57). Results showed no improvement in OS, which was the primary endpoint, despite improved RRs and PFS. However, there was a non-statistically significant trend toward improved survival for the addition of bevacizumab to chemotherapy (12.1 vs. 10.1 months, P=0.1002). In a planned subset analysis, there did appear to be more benefit for European and Pan-American patients than Asian patients.
The contention that gastric cancer in East Asia is fundamentally different than in the rest of the world was bolstered by a biomarker analysis of the AVAGAST study (58). Baseline VEGF-A levels as well as baseline levels of neuropilin-1, transmembrane glycoproteins involved in angiogenesis and tumor growth, were found to be prognostic and predictive. In patients who received chemotherapy alone, higher VEGF-A level and lower neuropilin-1 expression were associated with shorter OS. In patients who received bevacizumab and chemotherapy, this same pattern was associated with greater benefit from the addition of bevacizumab. Noticeably, the relationship between baseline VEGF-A levels and benefit from bevacizumab was seen only in non-Asian patients. Additional evidence to suggest that bevacizumab may offer greater benefits to a Western population comes from the two phase II studies performed by our group that paved the way for the AVAGAST study (59,60). In both of these studies, the addition of bevacizumab to different chemotherapy regimens improved survival outcomes significantly, compared to historical control.
Anti-VEGF receptor 2 mAb (ramucirumab)
Momentum for the evaluation of anti-VEGF therapies in EGC was halted after the AVAGAST trial until the results of the phase III REGARD study were made available (61). Three hundred and fifty-five patients with EG adenocarcinoma with prior progression on first-line fluoropyrimidine and/or platinum based chemotherapy were randomized 2:1 to receive ramucirumab, an antibody against VEGFR2 (which is the main receptor for VEGF-A), vs. placebo. PFS (2.1 vs. 1.3 months, HR =0.48, P<0.0001) and OS (5.2 vs. 3.8 months, HR =0.78, P=0.047) were improved in the ramucirumab arm. There was no difference in response rate observed between ramucirumab and placebo (3% in both arms). Hypertension was more common with the use of ramucirumab, being reported in 8% of patients. On the basis of this study, the FDA approved ramucirumab as second-line monotherapy in April 2014.
In addition, the phase III RAINBOW study evaluated ramucirumab in combination with second-line chemotherapy. The trial enrolled 665 patients and randomized them to paclitaxel with or without ramucirumab (62). Median OS was superior for the ramucirumab/paclitaxel arm (9.6 vs. 7.4 months, P=0.017); RRs (28% vs. 16%, P=0.0001) and PFS (4.4 vs. 2.9 months, P<0.0001) were also improved. When combined with paclitaxel, the only other toxicity observed in addition to hypertension was a higher incidence of grade 3/4 neutropenia (41% vs. 19%) but not a higher rate of neutropenic fever (3% vs. 2%). The survival in the combination arm compares very favorably with other phase III studies that have shown a survival benefit for 2nd-line taxane [5.2 months in the U.K. COUGAR-02 study (63) and 5.3 months in a Korean study where patients received either docetaxel or irinotecan (64)]. Survival in these chemotherapy-only studies also suggests that single-agent ramucirumab conveys a similar survival benefit as single-agent taxane or irinotecan.
A subset analysis that compared 140 Japanese patients on this study with 398 patients from Western countries has been published (65). It showed that the Japanese patients appeared to derive more benefit in terms of improvements in RR and PFS with the addition of ramucirumab. The RRs of Japanese patients who received ramucirumab/paclitaxel vs. paclitaxel was 41% vs. 19% respectively (P=0.0035) compared to 27% vs. 13% respectively (P=0.0004) for Western patients. Similarly, the median PFS for Japanese patients who received ramucirumab/paclitaxel vs. paclitaxel was 5.6 vs. 2.8 months (HR =0.503, P=0.0002) compared to 4.2 vs. 2.8 months in Western patients (HR =0.631, P<0.0001). However, there was no OS benefit in the Japanese patients (11.4 vs. 11.5 months, P=0.5113), whereas Western patients did have improved OS (8.6 vs. 5.9 months, P=0.0050). This may have been because of the increased use of post-discontinuation therapy in Japanese patients compared to Western patients (75% vs. 37%). When only Japanese patients who did not receive additional therapy upon progression were analyzed, those who received ramucirumab did have superior OS (9.6 vs. 4.3 months, HR =0.338, 95% CI: 0.124−0.922). Whether Japanese patients were able to receive more second-line and beyond therapy because of a more favorable tumor biology that led to a slower decline in performance status, superior oncologic care or some other factor is not known.
On the other hand, the results of a randomized phase II study of FOLFOX chemotherapy (bolus and infusional 5-FU/leucovorin/oxaliplatin) with or without ramucirumab as first-line therapy in esophageal and gastric adenocarcinoma were negative (66). There was a higher rate of premature discontinuation observed in the ramucirumab arm. The reasons for stopping treatment are not clear and did not implicate a specific AE. This raises the possibility that the addition of ramucirumab to mFOLFOX6 led to increased toxicity. In an unplanned subset analysis, there was a suggestion that patients with GEJ/gastric tumors who came off-study for a reason other that disease progression derived more benefit than patients with esophageal tumors. It seems doubtful that this represents a meaningful distinction since it is difficult clinically to determine if the primary tumor truly arises from the esophagus or contacts the GEJ.
A number of these unanswered questions will be addressed in the phase III study evaluating first-line fluoropyrimidine/cisplatin with or without ramucirumab, which has completed accrual (RAINFALL; clinicaltrials.gov NCT02314117).
Anti-VEGF TKIs
Sunitinib is an oral TKI that has activity against multiple receptor kinases including VEGFR. It has undergone disappointing single-agent assessment in EGC. In a German study, 51 patients with progression on at least one prior chemotherapy regimen received sunitinib (67). Unfortunately, both the RR (3.9%) and median PFS (1.28 months) were low. In another multinational study of GEJ and gastric adenocarcinoma (94% of patients had gastric tumors), sunitinib administered as second-line therapy was associated with similarly low RR (2.6%), PFS (2.3 months) and OS (6.8 months) (68). Similarly, disappointing results have been noted in a randomized phase II study of second-line docetaxel chemotherapy with or without sunitinib in 105 Korean gastric cancer patients with prior progression on a fluoropyrimidine/platinum regimen (69). While RRs were improved in the combination therapy arm (41% vs. 14%, P=0.0002), there was only a numerically but not statistically significant improvement in TTP (3.9 vs. 2.6 months, P=0.206) with the addition of sunitinib. Finally, sunitinib has also been studied in the adjuvant setting in a phase II study of pre-operative chemoradiation followed by surgery and adjuvant sunitinib (70). Of 70 patients who were initially enrolled, 25% did not start sunitinib and, of those who received adjuvant therapy, 28% discontinued therapy for toxicity. Survival did not appear to be clearly improved compared to historical controls.
Sorafenib is another multi-target TKI with activity against several targets, including VEGFR-1 and -2. In contrast to the negative sunitinib studies, two encouraging single-arm studies combining sorafenib with chemotherapy have been performed. In the first study sorafenib was combined with docetaxel/cisplatin as first-line therapy in 44 patients with metastatic or unresectable gastric and GEJ adenocarcinoma (74% had GEJ tumors) (71). The RR was 41%, median PFS was 5.8 months, while median OS was 13.6 months. Despite these promising outcomes, toxicity on this trial appeared to be substantial: 91% of patients developed grade 3 treatment-related toxicity. The most common grade ≥3 toxicity was neutropenia (64%) and there were 2 treatment-related deaths (5%). Twenty-three percent of patients discontinued treatment because of toxicity.
A Korean phase I study, involving 21 patients with advanced gastric/GEJ cancer, evaluated escalating doses of sorafenib in combination with capecitabine/cisplatin as first-line therapy (72). The RR was 62.5%, with a median PFS and OS of 10.0 and 14.7 months respectively. Again, hematologic toxicity was substantial (grade 3/4 neutropenia in 66.7% of patients but neutropenic fever in only 1 patient). At MSKCC, we completed a single-arm phase II study of sorafenib (NCT00917462) in patients with advanced chemorefractory esophageal cancer (with median of two prior therapies) (73). In 34 evaluable patients, the median PFS was 3.6 months and median OS was 8.8 months. One patient experienced a durable complete response that is ongoing at 40+ months. Overall, 20 of 34 patients were progression-free at 2 months, which met the statistical end-point for this study.
Apatinib, an anti-VEGFR2 TKI, was evaluated in a phase III study in 267 Chinese GEJ and gastric adenocarcinoma patients who had received ≥2 prior chemotherapy regimens (74). Patients were randomized to receive either apatinib or placebo. Median OS was significantly improved in the apatinib group (6.5 vs. 4.7 months, HR =0.71, P=0.0149), as was PFS (2.6 vs. 1.8 months, HR =0.444, P<0.001). Responses in the apatinib group were rare (2.84% vs. 0% for placebo-treated patients). Toxicities were consistent with those of other anti-VEGFR TKIs. On the basis of this study, apatinib is approved in China for this indication.
Additional evidence that anti-VEGFR TKIs are active in non-Asian patients with EG adenocarcinoma comes from a randomized phase II INTEGRATE study of regorafenib, another VEGFR2 TKI, which randomized 152 GEJ and gastric adenocarcinoma patients from Australia, New Zealand, South Korea and Canada who had received ≤2 prior regimens in a 2:1 fashion to regorafenib vs. placebo (75). The primary endpoint was PFS, which was improved in the regorafenib arm (2.6 vs. 0.9 months, HR =0.40, P<0.001). There was a trend toward improved OS (5.8 vs. 4.5 months, HR =0.74, P=0.147) and an OS benefit may have been obscured by the fact that the study permitted cross-over to regorafenib at the time of progression. The grade 3/4 toxicity rate appears to be similar to those of apatinib. Interestingly, the benefit for regorafenib seemed to be greater in the South Korean than other patients (HR: 0.12 vs. 0.61, P<0.001). On the basis of this study, a phase III study (INTEGRATE II, clinicaltrials.gov NCT02773524) has recently opened in the U.S.
It is worth noting that the hazard ratio for PFS and OS are virtually identical for apatinib and regorafenib (and very similar for single-agent second-line ramucirumab), suggesting a robust anti-angiogenic class effect for these drugs in EG adenocarcinoma. It is also important to note that none of the patients in these studies received prior ramucirumab so it is unclear if VEGFR TKIs will have activity in this increasingly common group of patients. INTEGRATE II will permit prior ramucirumab and will hopefully definitively answer this question. For now, especially given its superior toxicity profile relative to the VEGFR TKIs, ramucirumab in the second-line setting remains standard.
mTOR inhibition
The mTOR serine-threonine kinase, is a downstream component of the phosphatidylinositol 3-kinase (PI3K)/Akt kinase pathway and has been identified as a new therapeutic target for many differing types of cancer. In gastric cancer, upregulation of this pathway has been correlated with a worse prognosis (76) and may contribute to chemotherapy resistance (77).
The oral mTOR inhibitor everolimus demonstrated promising efficacy in a phase II study in 53 patients with advanced gastric cancer whose disease progressed after one or two previous chemotherapy lines (78). The disease control rate was 54.7%, median PFS was 2.7 months, and median OS was 10.1 months. Based on these results, everolimus was investigated in the phase III GRANITE-1 study, which treated patients with progression on ≤2 prior chemotherapy regimens (79). There was no improvement in the primary endpoint of OS (median OS: 5.4 vs. 4.3 months, HR =0.90; 95% CI: 0.75 to 1.08; P=0.124) or PFS (median PFS: 1.7 vs. 1.4 months, HR =0.66; 95% CI: 0.56 to 0.78).
The ability of everolimus to improve the efficacy of second-line therapy with paclitaxel, was investigated in a randomized phase III study, which enrolled 300 patients (80). Results were presented in abstract form earlier this year, which showed there was no significant difference in median PFS (2.2 vs. 2.07 months, HR =0.88, P=0.3) or median OS (6.1 vs. 5.1 months, HR =0.92, P=0.48). The combination of paclitaxel and everolimus was tolerated; however, there was less mucositis (15.8% vs. 37.2%), neutropenia (13.0% vs. 27.6%), leukopenia (11.6% vs. 21.4%), thrombocytopenia (2.1% vs. 14.5%) and fever (10.3% vs. 20.7%) in the placebo arm. Although everolimus failed to improve survival in pretreated patients with EGC, future biomarker studies will be performed in an attempt to identify subgroups that may derive a benefit.
MET inhibition
The TK receptor for HGF is MET, which has become a target of interest for EGC (81). The MET oncogene is amplified in 10–15% of gastric cancers (82) and the MET protein is overexpressed by IHC in approximately 40% of EGCs, which has been shown to be a poor prognostic factor in EGC (83,84).
Much of the interest in inhibition of this pathway comes from the positive results of a phase II study of the monoclonal antibody against HGF, rilotumumab (85). In this study rilotumumab was combined with chemotherapy and met its primary endpoint of improving PFS (5.6 vs. 4.2 months, HR =0.64, 80% CI: 0.48–0.85). In a subset analysis, patients whose tumors overexpressed MET (as determined by IHC) appeared to derive more benefit from rilotumumab therapy in terms of PFS (6.9 vs. 4.6 months, HR =0.53, 80% CI: 0.25–1.13) and OS (11.1 vs. 5.7 months, P=0.012). On the basis of these results, a global phase III trial (RILOMET-1) was commenced for patients who over-expressed MET by IHC (86). The study enrolled 609 previously untreated patients and randomized them to ECX with or without rilotumumab. The study was stopped early due a deleterious effect of rilotumumab, with OS (placebo, 11.5 vs. 9.6 months, rilotumumab), PFS (5.7 months in both cohorts) and ORR (placebo, 39% vs. 30% rilotumumab) favoring the placebo. No subgroups appeared to benefit from rilotumumab, including those with high expression of MET.
Another monoclonal antibody targeting MET called onartuzumab, failed to demonstrate an improvement in PFS when combined with FOLFOX chemotherapy in a phase II study in both an unselected population or in a MET IHC-positive population (87). As a consequence of these negative results the phase III study was stopped after 562 patients were enrolled. OS (11.3 vs. 11 months) and PFS (6.8 vs. 6.7 months) were similar in the placebo and onartuzumab arms (88). There was no benefit seen in the group of patients with high expression of MET by IHC.
Anti-MET TKIs
Similarly to MET monoclonal antibody directed therapy, early evaluations of MET TKIs have been underwhelming. A number of agents have been tested including, crizotinib, foretinib and tivantinib, all with negative results.
Tivantinib has been tested in combination with FOLFOX chemotherapy in a phase II study (89). The combination treatment showed a RR and PFS in the range of historical controls for first-line FOLFOX therapy. However, 3 patients did have extended time on study (16+ months). There are currently no active trials investigating this agent further in advanced EGC. Another agent called AMG 337, showed very encouraging results in a phase 1 study of MET inhibition in solid tumors (90). The ORR was 62% (8/13) in patients with EGC. This agent subsequently entered phase II testing in advanced EGC but was terminated following an interim review of efficacy and safety data (NCT02016534); results have yet to be presented.
The results of the trials targeting MET in EGC have been disappointing, raising doubts about the usefulness of further testing agents that inhibit the MET pathway in this disease. One possible explanation for the negative results with MET inhibition is that patient selection has not been correct. Patients have been selected on the basis of IHC, which does not distinguish between MET overexpression and MET amplification.
The PARP inhibition
Human cells have at least five primary pathways of DNA repair, one of which is double-strand break recombinational repair, which includes both non-homologous and homologous recombinational (HR) repair (91). The PARP are a family of enzymes, which are activated by DNA damage and which facilitate DNA repair (92). PARP inhibitors trap inactivated PARP onto single-strand DNA breaks, preventing repair and leading to double strand DNA breaks (93). BRCA-mutated cells are incapable of HR, therefore PARP inhibition results in genomic instability and cell death. Consistent with this, olaparib, an oral PARP inhibitor has demonstrated significant clinical benefit in patients with BRCA-mutated tumors, most notably ovarian and breast cancer. It is FDA approved for germline BRCA mutated advanced ovarian cancer that has received three or more prior lines of chemotherapy. The OlympiAD trial was published recently, which showed that olaparib improved response rates and prolonged PFS by 3 months compared to standard chemotherapy in patients with germline BRCA mutated metastatic breast cancer who had received ≤2 lines of therapy (94). However, there is a low prevalence of BRCA mutations in gastric cancer. Low ataxia telangiectasia mutated (ATM) levels have been shown to be associated with olaparib sensitivity (95). In addition, olaparib sensitivity was seen in gastric cancer cell lines deficient in ATM (96) with approximately 13% to 22% of tumors from patients with gastric cancer having low or undetectable ATM expression (97). Similar to BRCA mutated cancers, tumors low in ATM are an attractive target for PARP inhibition.
A phase II study investigated the oral PARP inhibitor olaparib combined with paclitaxel versus paclitaxel alone as second-line therapy in Asian patients with advanced gastric cancer (98). One hundred and twenty-four patients were randomly assigned to paclitaxel with olaparib vs. placebo. The screening prevalence of ATM low patients was 14%. Olaparib did not lead to a significant improvement in PFS, the primary endpoint. It did however, significantly improve OS vs. placebo in both the overall population (13.1 vs. 8.3 months, P=0.005) and the ATM low population (not reached vs. 8.2 months, P=0.002).
However, in the phase III GOLD trial, olaparib/paclitaxel did not meet its primary endpoint of improving OS in patients with advanced gastric cancer (99). The study randomized 525 Asian patients to olaparib plus weekly paclitaxel or paclitaxel alone. The co-primary endpoints were OS in all patients and OS in patients with ATM protein–negative tumors. Median OS was 8.8 months in the olaparib arm and 6.9 months in the placebo arm (HR =0.79, P=0.0262). In ATM protein-negative patients (n=94), OS was 12.0 vs. 10.0 months, respectively (HR =0.73, P=0.2458). The lack of benefit was seen in both the entire study population and in patients selected for ATM protein negativity.
Anti-CLDN 18.2 targeted therapy
Claudins are a family of proteins, which play a role in maintaining tight cell junctions. Different claudin subtypes are expressed on different tissues. Claudin 18 isoform 2 (CLDN18.2) expression in normal tissues is restricted to cells of the gastric mucosa and is absent from other healthy tissues (100). However, CLDN 18.2 is expressed in 70% of primary gastric adenocarcinomas and its metastases (101). It has also been shown to be expressed in a number of other cancers, including pancreatic (50%), esophagus (30%) and NSCLC (25%) (101). The identification of CLDN18.2 on gastric cancer became a potential target with the ensuing development of a monoclonal antibody against CLDN18.2, claudiximab (IMAB362).
Claudiximab is a chimeric IgG1 antibody directed against CLDN18.2. Upon administration, claudiximab specifically binds to CLDN18.2, which exerts its mechanism of action by activation of complement-dependent cellular cytotoxicity (CDCC) and antibody-dependent cellular cytotoxicity (ADCC). Additionally, it may interfere with the biological function of CLDN18.2 in cancer cells, resulting in antiproliferative and proapoptotic effects, the exact mechanism of which is not precisely known at this time.
A phase I trial investigated claudiximab in combination with zoledronic acid and interleukin-2 in patients with CLDN18.2+ in EGCs (102). Twenty-eight patients with metastatic disease who had received >1 line of therapy were enrolled. There were 20 evaluable patients, 11 of which achieved disease control, 10 patients had stable disease and 1 patient had a PR. An encouraging PFS (12.7 weeks) and OS (40 weeks) was observed.
The phase II (MONO) study, investigated claudiximab as a single agent in 54 patients with advanced disease (103). Results showed a response rate of 10%, and the disease control rate was 30%.
Most recently, results of the randomized phase II (FAST) study were presented (104). This study investigated claudiximab as first line therapy in patients with metastatic gastric and GEJ cancer. Patients were eligible if their tumors expressed CLDN18.2 (defined as ≥2+ in ≥40% tumor cells by IHC). Of the 739 patients screened, 352 (48%) tested CLDN18.2 positive. One hundred and sixty-one of these patients were randomized to EOX chemotherapy (epirubicin/oxaliplatin/capecitabine) with or without claudiximab. The combination of claudiximab plus EOX significantly improved PFS (median 7.9 vs. 4.8 months; HR =0.47; P = 0.0001) and OS (median 13.3 vs. 8.4 months; HR 0.51, P vs. 5.6 months; HR =0.36; P = 0.0005; OS, 16.6 vs. 9.3 months; HR 0.44, P vs. 25%). Vomiting was the most frequent toxicity reported in the claudiximab arm (grade 1/2 vomiting rates were 55.8% vs. 34%, while grade 3/4 vomiting rates were 10.4% vs. 3%). The results for claudiximab are very promising and results of the confirmatory phase III trial, which is due to open later this year, will be eagerly anticipated. In addition to combining claudiximab with chemotherapy, combination of claudiximab with immune stimulating therapies e.g., checkpoint inhibitors or agents that stimulate natural killer (NK) cells, will be worth pursuing, given that claudiximab exerts its main effect through stimulation of CDCC and ADCC.
Conclusions
Targeted therapy for EGC has seen an unprecedented growth as a result of our better understanding of the molecular mechanisms involved in esophagogastric tumorigenesis. Demonstration of the benefit of adding trastuzumab to first-line chemotherapy for patients with Her2 positive tumors was an important milestone. Since then, strategies that have been successful in Her2-positive breast cancer, have failed in EGC. The one remaining study, the phase III JACOB study with pertuzumab, has yet to be presented.
More recently, the approval of ramucirumab as second-line monotherapy has expanded options for our patients. However, phase III studies of anti-EGFR mAbs and TKIs have been uniformly negative and have severely dampened enthusiasm for further evaluation of any currently available anti-EGFR therapy in this disease. Similarly, phase III studies investigating both mTOR and MET inhibition have resulted in negative findings and it is hard to justify further testing of these targets in EGC going forward.
Despite a negative phase III study of PARP inhibition with olaparib, PARP targeting warrants further investigation, possibly in combination with other targeted therapies or immune checkpoint inhibition and in a biomarker-selected population. The identification of CLDN18.2 and its targeting with claudiximab is very promising and accrual to the phase III will likely be swift.
In the future, these and other trials aim to clarify the role of these targeted therapies, both as single agents in combination with established treatments in both the metastatic and peri-operative setting. It is hoped that these biologically-plausible trials, which incorporate informative correlative components, will further our understanding of the biology of EGCs and add to the armamentarium available to treat them.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Dulak AM, Schumacher SE, van Lieshout J, et al. Gastrointestinal adenocarcinomas of the esophagus, stomach, and colon exhibit distinct patterns of genome instability and oncogenesis. Cancer Res 2012;72:4383-93. [Crossref] [PubMed]
- Deng N, Goh LK, Wang H, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 2012;61:673-84. [Crossref] [PubMed]
- van Grieken NC, Aoyama T, Chambers PA, et al. KRAS and BRAF mutations are rare and related to DNA mismatch repair deficiency in gastric cancer from the East and the West: results from a large international multicentre study. Br J Cancer 2013;108:1495-501. [Crossref] [PubMed]
- Karamouzis MV, Grandis JR, Argiris A. Therapies directed against epidermal growth factor receptor in aerodigestive carcinomas. JAMA 2007;298:70-82. [Crossref] [PubMed]
- Wilkinson NW, Black JD, Roukhadze E, et al. Epidermal growth factor receptor expression correlates with histologic grade in resected esophageal adenocarcinoma. J Gastrointest Surg 2004;8:448-53. [Crossref] [PubMed]
- Kitagawa Y, Ueda M, Ando N, et al. Further evidence for prognostic significance of epidermal growth factor receptor gene amplification in patients with esophageal squamous cell carcinoma. Clin Cancer Res 1996;2:909-14. [PubMed]
- Itakura Y, Sasano H, Shiga C, et al. Epidermal growth factor receptor overexpression in esophageal carcinoma. An immunohistochemical study correlated with clinicopathologic findings and DNA amplification. Cancer 1994;74:795-804. [Crossref] [PubMed]
- Gibault L, Metges JP, Conan-Charlet V, et al. Diffuse EGFR staining is associated with reduced overall survival in locally advanced oesophageal squamous cell cancer. Br J Cancer 2005;93:107-15. [Crossref] [PubMed]
- Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:481-9. [Crossref] [PubMed]
- Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:490-9. [Crossref] [PubMed]
- Lorenzen S, Schuster T, Porschen R, et al. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2009;20:1667-73. [Crossref] [PubMed]
- Moehler MH, Thuss-Patience PC, Brenner B, et al. Cisplatin/5-FU (CF) +/- panitumumab for patients with non-resectable, advanced, or metastatic esophageal squamous cell cancer: An open-label, randomized AIO/TTD/BDGO/EORTC phase III trial (POWER). J Clin Oncol 2017;35:abstr 4011.
- Crosby T, Hurt CN, Falk S, et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol 2013;14:627-37. [Crossref] [PubMed]
- Suntharalingam M, Winter K, Ilson DH, et al. RTOG 0436: A phase III trial evaluating the addition of cetuximab to paclitaxel, cisplatin, and radiation for patients with esophageal cancer treated without surgery. J Clin Oncol 2014;32:abstr LBA6.
- Rao S, Starling N, Cunningham D, et al. Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: a randomised, multicentre open-label phase II study. Ann Oncol 2010;21:2213-9. [Crossref] [PubMed]
- Satoh T, Lee KH, Rha SY, et al. Randomized phase II trial of nimotuzumab plus irinotecan versus irinotecan alone as second-line therapy for patients with advanced gastric cancer. Gastric Cancer 2015;18:824-32. [Crossref] [PubMed]
- Herbst RS, Fukuoka M, Baselga J. Gefitinib--a novel targeted approach to treating cancer. Nat Rev Cancer 2004;4:956-65. [Crossref] [PubMed]
- Janmaat ML, Gallegos-Ruiz MI, Rodriguez JA, et al. Predictive factors for outcome in a phase II study of gefitinib in second-line treatment of advanced esophageal cancer patients. J Clin Oncol 2006;24:1612-9. [Crossref] [PubMed]
- Ferry DR, Anderson M, Beddard K, et al. A phase II study of gefitinib monotherapy in advanced esophageal adenocarcinoma: evidence of gene expression, cellular, and clinical response. Clin Cancer Res 2007;13:5869-75. [Crossref] [PubMed]
- Ferry DR, Dutton SJ, Mansoor W, et al. Phase III multi-centre, randomised, double-blind, placebo-controlled trial of gefitinib versus placebo in esophageal cancer progressing after chemotherapy, COG (Cancer Oesophagus Gefitinib). Ann Oncol 2012;23:LBA20.
- Ilson DH, Kelsen D, Shah M, et al. A phase 2 trial of erlotinib in patients with previously treated squamous cell and adenocarcinoma of the esophagus. Cancer 2011;117:1409-14. [Crossref] [PubMed]
- Dragovich T, McCoy S, Fenoglio-Preiser CM, et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol 2006;24:4922-7. [Crossref] [PubMed]
- Casalini P, Iorio MV, Galmozzi E, et al. Role of HER receptors family in development and differentiation. J Cell Physiol 2004;200:343-50. [Crossref] [PubMed]
- Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest 2001;19:554-68. [Crossref] [PubMed]
- al-Kasspooles M, Moore JH, Orringer MB, et al. Amplification and over-expression of the EGFR and erbB-2 genes in human esophageal adenocarcinomas. Int J Cancer 1993;54:213-9. [Crossref] [PubMed]
- Chan DS, Twine CP, Lewis WG. Systematic review and meta-analysis of the influence of HER2 expression and amplification in operable oesophageal cancer. J Gastrointest Surg 2012;16:1821-9. [Crossref] [PubMed]
- Yoon HH, Shi Q, Sukov WR, et al. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clin Cancer Res 2012;18:546-54. [Crossref] [PubMed]
- Brien TP, Odze RD, Sheehan CE, et al. HER-2/neu gene amplification by FISH predicts poor survival in Barrett's esophagus-associated adenocarcinoma. Hum Pathol 2000;31:35-9. [Crossref] [PubMed]
- Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes--a systematic review. Int J Cancer 2012;130:2845-56. [Crossref] [PubMed]
- Janjigian YY, Werner D, Pauligk C, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann Oncol 2012;23:2656-62. [Crossref] [PubMed]
- Okines AF, Thompson LC, Cunningham D, et al. Effect of HER2 on prognosis and benefit from peri-operative chemotherapy in early oesophago-gastric adenocarcinoma in the MAGIC trial. Ann Oncol 2013;24:1253-61. [Crossref] [PubMed]
- Terashima M, Kitada K, Ochiai A, et al. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res 2012;18:5992-6000. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Shah MA, Xu RH, Bang YJ, et al. HELOISE: Phase IIIb Randomized Multicenter Study Comparing Standard-of-Care and Higher-Dose Trastuzumab Regimens Combined With Chemotherapy as First-Line Therapy in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma. J Clin Oncol 2017;35:2558-67. [PubMed]
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91. [Crossref] [PubMed]
- Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017;18:640-53. [Crossref] [PubMed]
- Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 2009;69:9330-6. [Crossref] [PubMed]
- Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004;5:317-28. [Crossref] [PubMed]
- Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109-19. [Crossref] [PubMed]
- Kang YK, Rha SY, Tassone P, et al. A phase IIa dose-finding and safety study of first-line pertuzumab in combination with trastuzumab, capecitabine and cisplatin in patients with HER2-positive advanced gastric cancer. Br J Cancer 2014;111:660-6. [Crossref] [PubMed]
- Clynes RA, Towers TL, Presta LG, et al. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 2000;6:443-6. [Crossref] [PubMed]
- Bang YJ, Giaccone G, Im SA, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol 2017;28:855-61. [PubMed]
- Krop IE, Beeram M, Modi S, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol 2010;28:2698-704. [Crossref] [PubMed]
- Iqbal S, Goldman B, Fenoglio-Preiser CM, et al. Southwest Oncology Group study S0413: a phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol 2011;22:2610-5. [Crossref] [PubMed]
- Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014;32:2039-49. [Crossref] [PubMed]
- Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC--A Randomized Phase III Trial. J Clin Oncol 2016;34:443-51. [Crossref] [PubMed]
- Oh DY, Lee KW, Cho JY, et al. Phase II trial of dacomitinib in patients with HER2-positive gastric cancer. Gastric Cancer 2016;19:1095-103. [Crossref] [PubMed]
- Folkman J. Angiogenesis and angiogenesis inhibition: an overview. EXS 1997;79:1-8. [Crossref] [PubMed]
- Shih CH, Ozawa S, Ando N, et al. Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res 2000;6:1161-8. [PubMed]
- Kleespies A, Guba M, Jauch KW, et al. Vascular endothelial growth factor in esophageal cancer. J Surg Oncol 2004;87:95-104. [Crossref] [PubMed]
- Kitadai Y, Haruma K, Tokutomi T, et al. Significance of vessel count and vascular endothelial growth factor in human esophageal carcinomas. Clin Cancer Res 1998;4:2195-200. [PubMed]
- Inoue K, Ozeki Y, Suganuma T, et al. Vascular endothelial growth factor expression in primary esophageal squamous cell carcinoma. Association with angiogenesis and tumor progression. Cancer 1997;79:206-13. [Crossref] [PubMed]
- Imdahl A, Bognar G, Schulte-Monting J, et al. Predictive factors for response to neoadjuvant therapy in patients with oesophageal cancer. Eur J Cardiothorac Surg 2002;21:657-63. [Crossref] [PubMed]
- Yoshikawa T, Tsuburaya A, Kobayashi O, et al. Plasma concentrations of VEGF and bFGF in patients with gastric carcinoma. Cancer Lett 2000;153:7-12. [Crossref] [PubMed]
- Maeda K, Chung YS, Ogawa Y, et al. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer 1996;77:858-63. [Crossref] [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Van Cutsem E, de Haas S, Kang YK, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119-27. [Crossref] [PubMed]
- Shah MA, Ramanathan RK, Ilson DH, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 2006;24:5201-6. [Crossref] [PubMed]
- Shah MA, Jhawer M, Ilson DH, et al. Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol 2011;29:868-74. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78-86. [Crossref] [PubMed]
- Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513-8. [Crossref] [PubMed]
- Shitara K, Muro K, Shimada Y, et al. Subgroup analyses of the safety and efficacy of ramucirumab in Japanese and Western patients in RAINBOW: a randomized clinical trial in second-line treatment of gastric cancer. Gastric Cancer 2016;19:927-38. [Crossref] [PubMed]
- Yoon HH, Bendell JC, Braiteh FS, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter Phase II trial. Ann Oncol 2016;27:2196-203. [Crossref] [PubMed]
- Moehler M, Mueller A, Hartmann JT, et al. An open-label, multicentre biomarker-oriented AIO phase II trial of sunitinib for patients with chemo-refractory advanced gastric cancer. Eur J Cancer 2011;47:1511-20. [Crossref] [PubMed]
- Bang YJ, Kang YK, Kang WK, et al. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs 2011;29:1449-58. [Crossref] [PubMed]
- Yi JH, Lee J, Lee J, et al. Randomised phase II trial of docetaxel and sunitinib in patients with metastatic gastric cancer who were previously treated with fluoropyrimidine and platinum. Br J Cancer 2012;106:1469-74. [Crossref] [PubMed]
- Knox JJ, Wong R, Darling GE, et al. Adjuvant sunitinib (Su) for locally advanced esophageal cancer (LAEC): Results of a phase II trial. J Clin Oncol 2011;29:abstr 4091.
- Sun W, Powell M, O'Dwyer PJ, et al. Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. J Clin Oncol 2010;28:2947-51. [Crossref] [PubMed]
- Kim C, Lee JL, Choi YH, et al. Phase I dose-finding study of sorafenib in combination with capecitabine and cisplatin as a first-line treatment in patients with advanced gastric cancer. Invest New Drugs 2012;30:306-15. [Crossref] [PubMed]
- Janjigian YY, Vakiani E, Ku GY, et al. Phase II Trial of Sorafenib in Patients with Chemotherapy Refractory Metastatic Esophageal and Gastroesophageal (GE) Junction Cancer. PLoS One 2015;10:e0134731. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Pavlakis N, Sjoquist KM, Martin AJ, et al. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J Clin Oncol 2016;34:2728-35. [Crossref] [PubMed]
- Yu G, Wang J, Chen Y, et al. Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of chinese patients with gastric cancer. Clin Cancer Res 2009;15:1821-9. [Crossref] [PubMed]
- Yu HG, Ai YW, Yu LL, et al. Phosphoinositide 3-kinase/Akt pathway plays an important role in chemoresistance of gastric cancer cells against etoposide and doxorubicin induced cell death. Int J Cancer 2008;122:433-43. [Crossref] [PubMed]
- Doi T, Muro K, Boku N, et al. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol 2010;28:1904-10. [Crossref] [PubMed]
- Ohtsu A, Ajani JA, Bai YX, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 2013;31:3935-43. [Crossref] [PubMed]
- Al-Batran SE, Riera-Knorrenschild J, Pauligk C, et al. A randomized, double-blind, multicenter phase III study evaluating paclitaxel with and without RAD001 in patients with gastric cancer who have progressed after therapy with a fluoropyrimidine/platinum-containing regimen (RADPAC). J Clin Oncol 2017;35:4. [Crossref] [PubMed]
- Jhawer M, Kindler HL, Wainberg Z, et al. Assessment of two dosing schedules of GSK1363089 (GSK089), a dual MET/VEGFR2 inhibitor, in metastatic gastric cancer (GC): Interim results of a multicenter phase II study. J Clin Oncol 2009;27:4502.
- Bachleitner-Hofmann T, Sun MY, Chen CT, et al. HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Mol Cancer Ther 2008;7:3499-508. [Crossref] [PubMed]
- Tuynman JB, Lagarde SM, Ten Kate FJ, et al. Met expression is an independent prognostic risk factor in patients with oesophageal adenocarcinoma. Br J Cancer 2008;98:1102-8. [Crossref] [PubMed]
- Nakajima M, Sawada H, Yamada Y, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer 1999;85:1894-902. [Crossref] [PubMed]
- Oliner KS, Tang R, Anderson A, et al. Evaluation of MET pathway biomarkers in a phase II study of rilotumumab (R, AMG 102) or placebo (P) in combination with epirubicin, cisplatin, and capecitabine (ECX) in patients (pts) with locally advanced or metastatic gastric (G) or esophagogastric junction (EGJ) cancer. J Clin Oncol 2012;30:4005.
- Cunningham D, Tebbutt NC, Davidenko I, et al. Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study. J Clin Oncol 2015;33:4000.
- Shah MA, Cho JY, Tan IB, et al. A Randomized Phase II Study of FOLFOX With or Without the MET Inhibitor Onartuzumab in Advanced Adenocarcinoma of the Stomach and Gastroesophageal Junction. Oncologist 2016;21:1085-90. [Crossref] [PubMed]
- Shah MA, Bang YJ, Lordick F, et al. METGastric: A phase III study of onartuzumab plus mFOLFOX6 in patients with metastatic HER2-negative (HER2-) and MET-positive (MET+) adenocarcinoma of the stomach or gastroesophageal junction (GEC). J Clin Oncol 2015;33:4012.
- Pant S, Patel MR, Kurkjian C, et al. A phase II study of the c-Met inhibitor tivantinib (tiv) in combination with FOLFOX for the treatment of patients (pts) with previously untreated metastatic adenocarcinoma of the distal esophagus, gastroesophageal (GE) junction, or stomach. J Clin Oncol 2015;33:4065.
- Kwak EL, LoRusso P, Hamid O, et al. Clinical activity of AMG 337, an oral MET kinase inhibitor, in adult patients (pts) with MET-amplified gastroesophageal junction (GEJ), gastric (G), or esophageal (E) cancer. J Clin Oncol 2015;33:1. [Crossref] [PubMed]
- Plummer R. Perspective on the pipeline of drugs being developed with modulation of DNA damage as a target. Clin Cancer Res 2010;16:4527-31. [Crossref] [PubMed]
- Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays 2004;26:882-93. [Crossref] [PubMed]
- Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol 2011;5:387-93. [Crossref] [PubMed]
- Robson M, Im SA, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 2017;377:523-33. [Crossref] [PubMed]
- Hodgson D, Mason H, Oplustilova L, et al. Activity of the PARP inhibitor olaparib in ATM deficient gastric cancer: From preclinical models to the clinic. Proc Am Assoc Cancer Res 2014;74:abstr 2398.
- Kubota E, Williamson CT, Ye R, et al. Low ATM protein expression and depletion of p53 correlates with olaparib sensitivity in gastric cancer cell lines. Cell Cycle 2014;13:2129-37. [Crossref] [PubMed]
- Kim HS, Kim MA, Hodgson D, et al. Concordance of ATM (ataxia telangiectasia mutated) immunohistochemistry between biopsy or metastatic tumor samples and primary tumors in gastric cancer patients. Pathobiology 2013;80:127-37. [Crossref] [PubMed]
- Bang YJ, Im SA, Lee KW, et al. Randomized, Double-Blind Phase II Trial With Prospective Classification by ATM Protein Level to Evaluate the Efficacy and Tolerability of Olaparib Plus Paclitaxel in Patients With Recurrent or Metastatic Gastric Cancer. J Clin Oncol 2015;33:3858-65. [Crossref] [PubMed]
- Bang Y, Boku N, Chin K, et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy: Phase III GOLD study. Ann Oncol 2016;27:1-36. [Crossref]
- Swisshelm K, Macek R, Kubbies M. Role of claudins in tumorigenesis. Adv Drug Deliv Rev 2005;57:919-28. [Crossref] [PubMed]
- Sahin U, Koslowski M, Dhaene K, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res 2008;14:7624-34. [Crossref] [PubMed]
- Sahin U, Al-Batran SE, Hozaeel W, et al. IMAB362 plus zoledronic acid (ZA) and interleukin-2 (IL-2) in patients (pts) with advanced gastroesophageal cancer (GEC): Clinical activity and safety data from the PILOT phase I trial. J Clin Oncol 2015;33:e15079.
- Trarbach T, Schuler M, Zvirbule Z, et al. 636Pefficacy and safety of multiple doses of imab362 in patients with advanced gastro-esophageal cancer: results of a phase II study. Annals of Oncology 2014;25:iv218. [Crossref]
- Al-Batran SE, Schuler MH, Zvirbule Z, et al. FAST: An international, multicenter, randomized, phase II trial of epirubicin, oxaliplatin, and capecitabine (EOX) with or without IMAB362, a first-in-class anti-CLDN18.2 antibody, as first-line therapy in patients with advanced CLDN18.2+ gastric and gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol 2016;34:LBA4001. [Crossref]


