The management of localized esophageal squamous cell carcinoma: Western approach
Introduction
Esophageal cancer is the sixth most common cause of cancer related deaths worldwide, accounting for almost 500,000 new cases and 400,000 deaths annually (1,2). The incidence of esophageal cancer differs considerably based on geographic variation. In the US about 17,000 new cases are diagnosed each year (3), whereas in China, there were 477,000 cases in 2015 (4). The incidence also varies with gender, with males having a 2.4-fold rate than females. The 5-year survival rates of esophageal cancer range from 12% to 20% (5), with poor outcomes partly arising from advanced stage at diagnosis, but also from the fact that esophageal cancers are inherently resistant to systemic therapy as a result of histological, molecular and etiological heterogeneity.
Most esophageal cancers fall into two main histologic categories: esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC). Less than 1% are sarcomas or small cell carcinomas, with a very rare known incidence of esophageal lymphoma, melanoma, or carcinoid (5). EAC tends to occur in the lower esophagus near the gastric junction, whereas ESCCs predominate in the upper to mid-esophagus. Aside from differences in the site-specific predilection, the molecular pathogenesis of the two subtypes is quite distinct. Most sentinel clinical studies have nonetheless not differentiated between the two subtypes, so that for purposes of therapy, the therapeutic approaches have remained similar. The Cancer Genome Atlas has however recently revealed significant and important differences in the molecular patterns using next generation sequencing (6), calling for an urgent need to develop treatment paradigms directed to the specific subtypes.
Here, we will review recent advances in our evolving understanding of the demographics, risk factors, molecular pathogenesis, staging, and treatment of ESCCs from a US and European perspective.
Demographics and risk factors
Until ca. 1995, ESCC was the most common esophageal cancer subtype in the US, accounting for >95% of all cases. Since then, EAC has increased to account for approximately 80% of all esophageal cancers in the US (5), a shift in demographics that is attributable to an absolute increase in the incidence of EAC coupled with a decline in ESCC incidence. In fact, the incidence of EAC among white American males and females has risen by more than 4.6- and 3.4-fold, respectively, between 1975 and 2004 (5). This increase in EAC relates primarily to an increase in obesity, gastroesophageal reflux disease (GERD), and a precursor pre-malignant lesion Barrett’s esophagus (7). In contrast, the decline in incidence of ESCC has likely arisen from the steady decrease in alcohol use and smoking, which are both known risk factors.
The incidence of ESCC continues to predominate worldwide and is greatest in certain countries in the Eastern hemisphere, notably from central Asia to northern Iran—often known as the “esophageal cancer belt” (8). While the reason for this geographical predominance is not completely understood, certain predisposing factors, including limited intake of fruits and vegetables, nutritional deficiencies, such as vitamins A, C, E, zinc, folate, and selenium, drinking beverages at high temperatures, and nitrosamines have been implicated (2).
Certain risk factors are not related to geography. Achalasia due to chronic stasis and inflammation increases the risk of esophageal cancer by 28-fold, although routine screening is not recommended due to an overall low incidence (7). Likewise, patients with tylosis, an autosomal dominant disorder, have a 1.5- to 2-fold chance of developing ESCC during their lifetime, with endoscopy being recommended every 1 to 3 years (9). The increased incidence of ESCC in tylosis is due to missense mutations in a gene encoding the rhomboid protease RHBDF2 on chromosome 17q25 (10). HPV infection has also been cited as a potential risk factor although genomic analysis (as discussed below) has not been able to verify this association (6).
Gene mutations in ESCC
Genetic abnormalities predisposing patients to esophageal cancer have until recently been poorly understood. The use of next generation whole exome and genome sequencing has contributed substantially to our evolving understanding of the molecular pathogenesis of ESCCs and EACs. Similar to certain other cancers, esophageal cancer can arise from driver mutations, such as genes encoding the EGFR family of tyrosine kinase receptors, or inactivation of tumor suppressor genes, such as TP53 (11). Amplification of the EGFR gene has been widely recognized in the pathogenesis of ESCC, with the extent of overexpression generally correlating with poor prognosis (12,13). Likewise, loss-of-function mutations in the p53 pathway comprising CDKN2A (p14), MDM2, TP53 and CDKN1A (p21) genes, or the pRb pathway comprising the CDKN2A (p16), CCND1 and RB1 (pRb) genes, has been implicated (11,14,15).
Inactivation of the INK4A/CDKN2A locus, overexpression of cyclin D1 and/or inactivation of RB1 all cause ESCC by deactivating the pRb1 pathway. In contrast, the p53 pathway can be inactivated through TP53 mutations, MDM2 overexpression or p14ARF inactivation. Other genetic associations found within the (Online Mendelian Inheritance in Man) OMIM database, a continuously updated catalog of human genes and genetic phenotypes, include TGFRB2, DLEC1, LZTS1, DEC1, RNF6, WWOX, and DCC genes (http://www.omim.org/entry/133239). Furthermore, using genome-wide association, loss of heterozygosity was noted in several loci including 3p, 5q, 9p, 9q and 13q (16).
Gene-environment interactions
While somatic mutations associated with ESCC continue to be catalogued (17,18), there is increased interest in interrogating associations between the remarkable array of environmental risk factors implicated in esophageal cancer and its genetic predispositions. An interesting association between consumption of hot beverages and p53-mediated esophageal cancer was documented almost two decades ago, one of the first gene-environment interactions in the evolution of ESCC (19). More recently, the locus C20ORF54 was found to be associated with ESCC in the Han Chinese population. This gene encodes a protein responsible for riboflavin transport, which is a known risk factor for ESCC. Likewise, it has been established that ALDH2 polymorphisms are associated with ESCC, and that alcohol users with both ALDH1A and ALDH1B risk alleles are at a 4-fold risk of ESCC than drinkers without the risk alleles (20). A more recent study using SNP array-based profiling allowed the subgrouping of smokers and alcohol consumers with ESCC based on specific mutational signatures (18).
Molecular subtyping
It has been difficult to molecularly define esophageal cancers beyond histopathologic differences, and ESCC and EAC have therefore been traditionally treated as one entity in clinical practice, with similar chemotherapy and radiation regimens being prescribed irrespective of histology. The aforementioned mutations, including those of the P53 and CDK gene families, are not able to differentiate ESCC from EAC. More recent attempts have therefore examined the full landscape of genomic alterations in ESCC and EAC using whole genome sequencing in an attempt to molecularly separate the two subtypes (2,6,17,21). For example, besides identifying new mutated genes in ESCC, such as FAT1, FAT2, KMT2D and ZNF750, there was a preponderance of somatic copy number variations in 180 ESCC samples (6). Likewise, in a small study using 30 EAC samples from 15 patients, a preponderance of A>C mutations and enrichment of the 5' bases has been noted, particularly in those from the gastroesophageal junction (17). A comparative genomic analysis was also performed on 11 EAC and 12 ESCC specimens and found a significant difference in the mutational spectrum with more indels in ESCCs, A:T>C:G transversions in EACs, and C:G>G:C transversions in ESCCs (2). Additionally, there were also differences found in North American versus Chinese ESCCs with a greater frequency of inactivating NOTCH1 mutations in North American ESCCs, suggesting distinct pathogenetic pathways based on environmental and genetic factors (2).
More informative has been a recent larger comprehensive analysis by the Cancer Genome Atlas Research Network that utilized resected tumor tissue from 90 ESCC and 72 EAC patients for integrated genome analysis, including whole exome sequencing, SNP arrays, DNA methylation profiling, and mRNA and miRNA sequencing (20). Several key findings emerged from these studies. On every platform utilized, despite common signatures, such as the deletion or silencing of CDKN2, there was a distinct molecular signature that could differentiate the two histologic phenotypes. For example, 76% of EAC showed increased E-cadherin signaling, while ESCCs displayed an upregulation of WNT, SYN and P63 pathways.
Integrated clustering further identified three distinct ESCC subtypes, namely ESCC1-3. ESCC1 tissue displayed alterations in the NRF2 pathway, which is crucial for adaptation to oxidative stressors. This latter finding suggests that this particular subtype may be more resistant than others to chemoradiotherapy. In contrast, ESCC3s harbored SMARCA4 mutations, but without similarity to other squamous head and neck cancers, suggesting that this particular subtype may be confined to esophageal squamous cells (20). The ESCC subtypes also showed geographical segregation; for example, tumors from Vietnamese patients were enriched for NRF2 mutations, indicative of a common oxidative stressor or a genetic predisposition in this population (20).
Prior reports have suggested that HPV, which has a role in cervical and head and neck squamous cell cancers, also contributes to ESCC. However, strikingly, HPV transcript levels resembled HPV-negative head and neck cancers (20). Along with histopathologic differences, ESCCs showed a molecular semblance to head and neck squamous cell carcinomas, rather than to EACs. The latter tumors molecularly resembled a distinct group of gastric tumors characterized by chromosomal instability (CIN), suggesting that gastric tumors and EACs may be grouped together a single entity, similar to colorectal adenocarcinoma (20). We envisage that genomic subtyping and its correlation with histologic subtype, will in the future impact clinical trial design utilizing predefined genetic subtypes to assign the most appropriate targeted therapeutics.
Diagnosis and staging
There are a number of staging systems used in the US and Europe for diagnosing and staging esophageal cancers (22,23). In general, staging initially occurs with endoscopic evaluation, followed by PET or CT or both to assess for metastases. PET/CT has shown to be most sensitive for detecting occult malignancy. If there is no evidence of metastatic involvement, endoscopic ultrasound is used to assess for tumor invasion and regional lymph node involvement. In Western populations, ESCC tends to metastasize within the thorax first, whereas EAC tends to metastasize to distant sites with the abdominal cavity (24).
The most commonly used staging guidelines in the West are the American Joint Committee on Cancer (AJCC) system and the Union for International Cancer Control (UICC). Clinically, there is a general demarcation between the tumors that invade the submucosa (T1b) and beyond, and those that are limited to the mucosa (T0 or T1a). High-grade dysplasia (T0) or intramucosal carcinoma (T1a) lesions can be resected endoscopically. However, T1b ESCCs generally require esophagectomy. This is contrast to T1b EAC, where in some cases there may only be superficial involvement of the submucosa and thus be amenable to endoscopic resection (5,24).
The 7th edition UICC and AJCC Updated TNM (tumor, node, metastases) Definitions was created in 2009 by the Worldwide Esophageal Cancer Collaboration (WECC) using a database of 4,673 esophagectomy patients who had not received induction or adjuvant therapy from 13 institutions in 5 countries within 3 continents. Important distinctions were made in this edition. First, T1a was defined as high-grade dysplasia, which included previously termed carcinoma in situ or all non-invasive neoplastic epithelium. Second, T4 was further differentiated as T4a and T4b. T4a encompassed resectable cancers that invade adjacent structures, such as pleura, pericardium or diaphragm, whereas T4b was unresectable cancer that had invaded adjacent structures, such as the aorta, vertebral body, and/or trachea. Third, regional lymph node involvement was made to encompass any para-esophageal node extending from peri-esophageal cervical nodes to celiac nodes. Fourth, previously subcategorized M0, M1a, M1b, MX were simplified to include M0 as having no distant metastases and M1 as having distant metastases.
Most importantly, the 7th edition also accounted for histologic cell type, histologic grade, and tumor location as important determinants for early stage grouping (24). This change was driven by a large study showing that the prognosis of ESCC patients who underwent surgery is based not only on T stage but also histology, grade, and location of the tumor (25). ESCC and EAC now have separate stage groupings for stages I and II. T1N1M0 ESCC was further differentiated by histologic grade: G1 (well differentiated) versus G2–3 (moderately to poorly-differentiated). In contrast, for T2N0M0 and T3N0M0 ESCC, subgrouping was based on both histologic grade and location of tumor (Table 1).
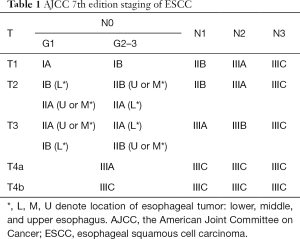
Full table
The 8th edition of the TNM staging guidelines are to be released in January 1, 2018 and will include further prognostic factors that will be specific to each histologic subtype. This edition has been implemented outside of the US by the UICC as of January 1, 2017. Notably, in this edition, an expanded database of 22,654 esophageal cancer patients was obtained from the 33 WECC institutions in 6 continents containing 13 countries. Thirty-nine variables were studied for future risk adjustment. The enhanced database has allowed for a more robust forest-based machine learning analysis through which risk-adjusted survival estimates have been analyzed for all patients.
Most importantly, the 8th edition presents separate classifications for clinical (cTNM), pathologic (pTNM) as described in Table 2, and post-neoadjuvant pathologic (ypTNM) staging (24). Significant changes are summarized in Table 3. First, the pathologic staging, pT1, has been subcategorized into pT1a and pT1b for both EAC and ESCC (Table 3). This sub-categorization, combined with grade, now correlates with two pathologic stage I categories for ESCC, namely pStage IA (pT1aN0M0G1) and pStage IB (pT1aN0M0G2-G3). Second, location has been removed as a subcategory for pT2N0M0 ESCC. Thus, lower and upper/middle esophageal tumors, which were previously staged separately, are now stage IB for grade 1 and stage IIA for grades 2 and 3. Third, G4, which connotes undifferentiated histologic grade, has been removed, and additional analysis is recommended to differentiate histologic cell type. Fourth, subgroup IIIC has been eliminated from the 7th edition, and stage IV has been subcategorized into stage IVA and stage IVB. Fifth, a more comprehensive regional lymph node map, encompassing para-esophageal lymph nodes from the upper esophageal sphincter to the celiac artery, has been instituted in the 8th edition. Finally, ypTNM stage groups have been introduced for patients who have undergone neoadjuvant therapy, resection, and pathologic review of the specimen. The ypTNM stage groups are the same for both histologic subtypes, unlike cTNM and pTNM. Clinical stage groups (cTNM) are also new to this edition and are based largely on imaging and not pathologic examination of the resected specimen.
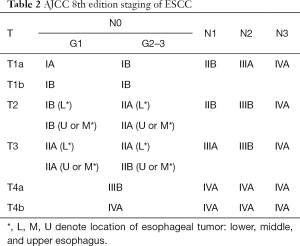
Full table
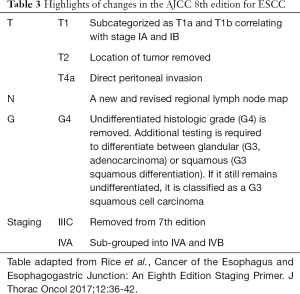
Full table
Treatment
As noted above, T0–T1a lesions may be endoscopically resected. Endoscopic mucosal resection (EMR) is widely available in the West, but endoscopic submucosal resection (ESD) tends to be limited to specialized centers (24). In patients with T1b or greater ESCC, however, neoadjuvant chemoradiation followed by esophagectomy is the favored approach in those considered as surgical candidates. The efficacy of the tri-modality approach was validated by the CROSS trial which compared preoperative chemoradiotherapy namely weekly paclitaxel (50 mg/m2) plus carboplatin plus concurrent radiotherapy (41.4 Gy over 5 weeks) versus upfront surgery alone in 366 patients (84 ESCC, 275 adenocarcinoma, 7 large-cell undifferentiated carcinoma) with resectable esophageal or gastro-esophageal junction cancers. The 5-year overall survival (OS) was greater in the tri-modality approach (47%) versus surgery alone (34%) (26).
Prior to the CROSS study there was much debate in the West about the optimal management of patients with stage II/III esophageal cancer with some centers favoring surgery alone or surgery followed by adjuvant therapy over the neoadjuvant approach. Many of the published studies were too small or were underpowered to answer this question definitively, and although the CROSS trial has many critics, it did show a significant benefit in patients with squamous cell carcinoma. Table 4 provides a summary of a select number of randomized controlled trials performed in the West comparing the tri-modality approach versus surgery alone (36).

Full table
There have been retrospective analyses that have demonstrated a survival benefit for the trimodality approach against chemoradiation alone (38). However, there is continued debate regarding the need for additional surgery after chemoradiation particularly with ESCCs. In contrast to EAC, complete endoscopic response rates are higher in ESCC following neoadjuvant chemoradiation. There are two reasons why, in contrast to ESCC, chemoradiation is almost always followed by surgery in patients with EACs. First, ESCC is more responsive to chemoradiation than EAC, and therefore has a greater likelihood of complete remission that is determined endoscopically. Second, ESCC are located in the upper to mid esophagus with close proximity to the trachea and major blood vessels, with surgical resection being more complicated and carrying a greater morbidity risk (5–10%). Additionally co-morbidities associated with ESCC predominantly a smoking related disease in the West, may also increase peri-operative risk (39).
The FFCD 9102 Study analyzed the utility of surgery after chemoradiation against chemoradiation alone. This study used 444 patients with locally advanced esophageal cancer (89% ESCC) who received induction chemoradiation with 5-fluouracil and cisplatin. Those with a partial response were either randomized to surgery or further chemoradiation (40). The tri-modality group had a lower rate of locoregional recurrence (34% versus 43%), but OS at 2 years was similar (34% versus 40%) (40). A subsequent subset analysis of 111 chemoradiation non-responders revealed that those who had undergone surgery had a greater median survival versus those who did not (17 versus 5.5 months), suggesting the need to stratify patients based on their initial response to chemoradiation. This is however not easy and endoscopic evaluation can be misleading (41). The addition of surgery was also evaluated in a large Cochrane meta-analysis, which reviewed eight trials encompassing 1,132 participants. This study concluded that definitive chemoradiation was equivalent to surgery in terms of both short- and long-term OS in patients responsive to induction chemoradiation; however, this result was less apparent in the adenocarcinoma subset (42).
In contrast, the management of early stage ESCC patients with endoscopic negative findings after chemoradiation remains less clear, and the need for locoregional control must be carefully balanced with surgical mortality. Studies have suggested that although the tri-modality approach in ESCC patients showed no benefit over the bi-modality approach in OS, it did show an increase in locoregional control. In one study, 172 patients with ESCC either received induction chemotherapy (5-fluouracil, etoposide, and cisplatin) followed by chemoradiation and resection versus induction chemotherapy followed by chemoradiation alone. The tri-modality group had an increased PFS (64% versus 41%) suggesting better locoregional control, but did not have a statistically significant difference in OS up to 10 years. Of note, the trimodality group did have a significantly greater treatment-related mortality (12.8% versus 3.5%) (43,44).
Neoadjuvant chemoradiation tends to be preferred in the west. There have been a few studies comparing chemoradiation to chemotherapy alone in the neoadjuvant setting. These studies have predominantly included adenocarcinoma and most have concluded higher rates of pCR in the chemoradiation arm. Chemotherapy alone in the neoadjuvant setting is however being compared to chemoradiation in locally advanced ESCC in the CMISG1701 study which is a multicenter, prospective, randomized, phase III clinical trial evaluating the efficacy of neoadjuvant chemoradiation plus minimally invasive esophagectomy versus neoadjuvant chemotherapy alone followed by surgery (45).
Immunotherapeutic approaches for localized ESCC
Similar to many other tumor types, immunotherapy is also being actively investigated in esophageal cancer and has shown promise. The ONO 12 (ATTRACTION-2) was a large multicenter, double-blind, randomized phase III trial that assessed nivolumab in metastatic gastric and esophageal cancer patients who were refractory to or intolerant to at least two prior chemotherapy regimens. This trial was the first phase III study to demonstrate an improved median OS for immunotherapy in esophagogastric cancer with patients in nivolumab group having an OS of 5.32 months (95% CI, 4.63–6.41) versus 4.14 months (95% CI, 3.42–4.86) in the placebo group (HR =0.63; P<0.001) (46). In addition, the 12-month OS in the nivolumab group was 26.6% (95% CI, 21.1–32.4) versus 10.9% (95% CI, 6.2–17.0). Objective response rate of 11.2% was seen in the nivolumab group versus 0% in the placebo group.
While many of the studies to date have only included EACs, there have been studies to support the potential use of nivolumab in patients with metastatic and locally advanced ESCCs. A recent phase II, open-label, single-arm, multicenter study in Japan administered nivolumab (3 mg/kg every 2 weeks) to 65 patients with advanced, treatment refractory ESCC. This study demonstrated an objective response in 17% of the patients with a median follow-up of 10.8 months (47).
While many completed or ongoing trials have investigated PD-1 inhibitors in the metastatic setting, there are now ongoing studies assessing the benefit of immunotherapy in the neoadjuvant and adjuvant setting for stage II/III ESCC. The CheckMate 577 trial is a large randomized phase III study of 760 patients post-trimodality therapy whose tumors did not have a complete pathologic response (ypT1 or ypN1 or greater disease); this trial includes both ESCCs and EACs. Patients are randomized in a 2:1 fashion to either nivolumab 240 mg IV every 2 weeks for 16 weeks, followed by 480 mg every 4 weeks for a maximum of 12 months or to placebo (surveillance is the standard of care). Smaller phase I and II studies are also being conducted to assess the efficacy and safety of nivolumab, pembrolizumab and durvalumab in the neoadjuvant setting.
The rationale for investigating the efficacy of immunotherapy in the neoadjuvant or adjuvant setting for stage II/III esophageal cancers appears stronger than its use in the metastatic setting, as successive chemotherapy regimens may have knocked down the immune system. Following chemoradiation, there is a significant upregulation of PDL-1 and other immune biomarkers in both ESCC and EAC subtypes—this suggests that localized disease may be sensitive to checkpoint blockade (48). Furthermore, it has been shown that following neoadjuvant chemoradiation, there is an induction of tumor infiltrating lymphocytes (TILs), perivascular lymphocytes, and tertiary lymphoid structures (49). These changes in the tumor microenvironment may support a role for checkpoint blockade in the neoadjuvant setting either in conjunction with chemoradiation or when administered sequentially.
Conclusions
Over the past decade there has been a significant shift in esophageal carcinoma histology from the ESCC to the EAC subtype, particularly in Western countries, likely resulting from changes in environmental cues, such as smoking cessation and the increased prevalence of obesity. Nonetheless, historically the two histologically distinct subtypes have been lumped together for purposes of chemoradiation and surgical treatment, although the benefit of surgical intervention in ESCC remains debated, particularly when an endoscopic response is achieved after chemoradiation. With the cataloging of somatic driver mutations, and the advent of genome sequencing, the emergence of the entire genetic and epigenetic landscape of esophageal cancer has become a reality. This, in turn, has led to attempts at distinguishing ESCC and EAC on the basis of molecular signatures, to which future therapies could potentially be targeted. Additionally, this genomic data could be utilized in future immunotherapy trials that are currently enrolling and may help identify predictive signatures of responsive disease. While ESCCs in the West have historically proven inherently resistant to systemic therapy after first line chemoradiation, we are now closer to realizing meaningful clinical benefits for our patients as a result of incremental improvements in our understanding of the molecular and immunological changes that occur in these heterogeneous tumors.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Kelly reports advisory boards for BMS, Eli Lilly, Astellas, EMD Serono, Gritstone Oncology, and AstraZeneca.
References
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, et al. The Global Burden of Cancer 2013. JAMA Oncol 2015;1:505-27. [Crossref] [PubMed]
- Agrawal N, Jiao Y, Bettegowda C, et al. Comparative genomic analysis of esophageal adenocarcinoma and squamous cell carcinoma. Cancer Discov 2012;2:899-905. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014;6:112-20. [Crossref] [PubMed]
- Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature 2014;505:612-3. [Crossref] [PubMed]
- Engel LS, Chow WH, Vaughan TL, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst 2003;95:1404-13. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- ASGE Standards of Practice Committee, Evans JA, Early DS, et al. The role of endoscopy in Barrett's esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc 2012;76:1087-94. [Crossref] [PubMed]
- Blaydon DC, Etheridge SL, Risk JM, et al. RHBDF2 mutations are associated with tylosis, a familial esophageal cancer syndrome. Am J Hum Genet 2012;90:340-6. [Crossref] [PubMed]
- Kuwano H, Kato H, Miyazaki T, et al. Genetic alterations in esophageal cancer. Surg Today 2005;35:7-18. [Crossref] [PubMed]
- Kitagawa Y, Ueda M, Ando N, et al. Further evidence for prognostic significance of epidermal growth factor receptor gene amplification in patients with esophageal squamous cell carcinoma. Clin Cancer Res 1996;2:909-14. [PubMed]
- Ozawa S, Ueda M, Ando N, et al. Prognostic significance of epidermal growth factor receptor in esophageal squamous cell carcinomas. Cancer 1989;63:2169-73. [Crossref] [PubMed]
- Olivier M, Eeles R, Hollstein M, et al. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat 2002;19:607-14. [Crossref] [PubMed]
- Parenti AR, Rugge M, Frizzera E, et al. p53 overexpression in the multistep process of esophageal carcinogenesis. Am J Surg Pathol 1995;19:1418-22. [Crossref] [PubMed]
- Hu N, Roth MJ, Polymeropolous M, et al. Identification of novel regions of allelic loss from a genomewide scan of esophageal squamous-cell carcinoma in a high-risk Chinese population. Genes Chromosomes Cancer 2000;27:217-28. [Crossref] [PubMed]
- Hu N, Kadota M, Liu H, et al. Genomic Landscape of Somatic Alterations in Esophageal Squamous Cell Carcinoma and Gastric Cancer. Cancer Res 2016;76:1714-23. [Crossref] [PubMed]
- Sawada G, Niida A, Uchi R, et al. Genomic Landscape of Esophageal Squamous Cell Carcinoma in a Japanese Population. Gastroenterology 2016;150:1171-82. [Crossref] [PubMed]
- Casson AG, Tammemagi M, Eskandarian S, et al. p53 alterations in oesophageal cancer: association with clinicopathological features, risk factors, and survival. Mol Pathol 1998;51:71-9. [Crossref] [PubMed]
- Wu C, Kraft P, Zhai K, et al. Genome-wide association analyses of esophageal squamous cell carcinoma in Chinese identify multiple susceptibility loci and gene-environment interactions. Nat Genet 2012;44:1090-7. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network, Analysis Working Group. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169-75. [Crossref] [PubMed]
- Berry MF. Esophageal cancer: staging system and guidelines for staging and treatment. J Thorac Dis 2014;6 Suppl 3:S289-97. [PubMed]
- Varghese TK Jr, Hofstetter WL, Rizk NP, et al. The society of thoracic surgeons guidelines on the diagnosis and staging of patients with esophageal cancer. Ann Thorac Surg 2013;96:346-56. [Crossref] [PubMed]
- Alsop BR, Sharma P. Esophageal Cancer. Gastroenterol Clin North Am 2016;45:399-412. [Crossref] [PubMed]
- Stahl M, Lehmann N, Walz MK, et al. Prediction of prognosis after trimodal therapy in patients with locally advanced squamous cell carcinoma of the oesophagus. Eur J Cancer 2012;48:2977-82. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Nygaard K, Hagen S, Hansen HS, et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg 1992;16:1104-9; discussion 10. [Crossref] [PubMed]
- Apinop C, Puttisak P, Preecha N. A prospective study of combined therapy in esophageal cancer. Hepatogastroenterology 1994;41:391-3. [PubMed]
- Le Prise E, Etienne PL, Meunier B, et al. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer 1994;73:1779-84. [Crossref] [PubMed]
- Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997;337:161-7. [Crossref] [PubMed]
- Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305-13. [Crossref] [PubMed]
- Lee JL, Park SI, Kim SB, et al. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol 2004;15:947-54. [Crossref] [PubMed]
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [Crossref] [PubMed]
- Hulshof MC, van Laarhoven HW. Chemoradiotherapy in tumours of the oesophagus and gastro-oesophageal junction. Best Pract Res Clin Gastroenterol 2016;30:551-63. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- McKenzie S, Mailey B, Artinyan A, et al. Improved outcomes in the management of esophageal cancer with the addition of surgical resection to chemoradiation therapy. Ann Surg Oncol 2011;18:551-8. [Crossref] [PubMed]
- Alexandrou A, Davis PA, Law S, et al. Squamous cell carcinoma and adenocarcinoma of the lower third of the esophagus and gastric cardia: similarities and differences. Dis Esophagus 2002;15:290-5. [Crossref] [PubMed]
- Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [Crossref] [PubMed]
- Vincent J, Mariette C, Pezet D, et al. Early surgery for failure after chemoradiation in operable thoracic oesophageal cancer. Analysis of the non-randomised patients in FFCD 9102 phase III trial: Chemoradiation followed by surgery versus chemoradiation alone. Eur J Cancer 2015;51:1683-93. [Crossref] [PubMed]
- Best LM, Mughal M, Gurusamy KS. Non-surgical versus surgical treatment for oesophageal cancer. Cochrane Database Syst Rev 2016;3:CD011498. [PubMed]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727-33. [Crossref] [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7. [Crossref] [PubMed]
- Tang H, Tan L, Shen Y, et al. CMISG1701: a multicenter prospective randomized phase III clinical trial comparing neoadjuvant chemoradiotherapy to neoadjuvant chemotherapy followed by minimally invasive esophagectomy in patients with locally advanced resectable esophageal squamous cell carcinoma (cT3-4aN0-1M0) (NCT03001596). BMC Cancer 2017;17:450. [Crossref] [PubMed]
- Kang YK, Satoh T, Ryu MH, et al. Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): A double-blinded, randomized, phase III trial. J Clin Oncol 2017;35:suppl 4S; abstract 2.
- Kojima T, Hara H, Yamaguchi K, et al. Phase II study of nivolumab (ONO-4538/BMS-936558) in patients with esophageal cancer: Preliminary report of overall survival. J Clin Oncol 2016;34:suppl 4S; abstract TPS175.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Thompson ED, Zahurak M, Murphy A, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 2017;66:794-801. [Crossref] [PubMed]


