Stereotactic body radiotherapy for early stage lung cancer—historical developments and future strategies
Introduction
Since the mid-1980s, efforts have been made to improve outcomes in non-small cell lung cancer (NSCLC) through escalating radiotherapy doses (1). However, attempts to dose escalate through either conventional fractionation or even altered fractionation has led to either disappointing tumour control or unacceptable toxicities (2-4). In the mid-1990s, the application of “intracranial radiosurgery” extra-cranially to treat small lung tumour targets was made possible with incremental technological advancements, starting first with the use of the rigid stereotactic body frame and then becoming mainstream when improvements in image guidance enabled frameless treatments and respiratory motion management. This was subsequently followed by a series of well-planned clinical studies in the early-2000s, which demonstrated efficacy and low rates of treatment-related toxicities (5-8). Since then, lung stereotactic body radiotherapy (SBRT) has firmly established itself as a standard treatment in early stage node negative medically inoperable NSCLC, effectively doubling biological effective dose (BED) and tumour control rates that were previously achieved with conventional radiotherapy (2). No other development in the management of NSCLC has had quite the same dramatic success. In this review, we will discuss the radiobiological principles underlying lung SBRT, technical considerations that are vital to its safe delivery, existing evidence supporting its use in various clinical settings and finally explore various strategies to optimise the therapeutic ratio in lung SBRT.
Search strategy
We searched the PubMed and MEDLINE databases for articles published in English from 1 January 2000 to 31 Dec 2016 with the keywords “conventional fractionation”, “stereotactic body radiotherapy”, “stereotactic ablative radiotherapy”, “dose escalation”, “biological effective dose”, “radiobiology”, “early stage”, “lung cancer”, “peripheral”, “central”, “toxicities”, “complications” “pneumonitis”, “Intensity Modulated Radiotherapy”, “Volumetric Modulated Arc Therapy”, “Proton therapy”, “molecular”, “genomics”, “biomarkers”. Articles were selected based on relevance, with priority given to highly cited articles, randomised clinical trials and articles written in English. Abstracts of main medical conferences were also included if survival and toxicity end-points were reported. Articles that were published before or after the search time frame were also included if they were widely referenced and highly regarded seminal work.
Radiobiology of Lung SBRT
SBRT is characterized by precision delivery of single large doses (generally ≥6 Gy) either in a single fraction or in a small number of fractions to a target volume (9). Delivery of radiation dose in this manner enables sharp escalation of BED as modelled by the classic linear quadratic (LQ) model resulting in better local tumour control probability (TCP) (2,10). The application of the LQ model to very large fraction sizes has been disputed due to in vitro and in vivo data suggesting an overestimation of cell killing at large single doses compared to more fractionated regimens (11). Furthermore, large radiation doses, similar to those used in SBRT, have been demonstrated to produce additional radiobiological effects including the induction of sphingomyelinase dependent ceramide-induced tumour endothelial cell apoptosis (12,13), vascular damage leading secondarily to tumour cell killing (14) as well as enhanced anti-tumour immune responses (15). However, correlation with actual outcome data for stage I NSCLC treated with typical lung SBRT dose-fractionations demonstrates accurate radiobiological modelling with both classic LQ model as well as a modified (16), “more realistic” version of the LQ model accounting for intra- and inter-tumour heterogeneity, therefore suggesting that additional radiobiological processes do not contribute significantly to cell killing in lung SBRT (17,18). This does not however mean that these additional radiobiological processes do not exist and they could yet be exploited in combination treatment strategies involving SBRT (19,20).
Technical considerations of lung SBRT
Precision delivery of high radiation doses to a moving tumour target in the lung requires respiratory motion control, dose construction with strict adherence to normal tissue dose constraints and treatment dose delivery with setup and target verifications (21-23).
Respiratory motion management
Management of respiratory motion is absolutely necessary both at simulation and subsequently during treatment delivery. During simulation, patients are often immobilised with a whole-body vacuum cushion with or without abdominal compression and respiration motion is mostly accounted for through the use of multiple CT scans taken at various points of the normal respiratory cycle, 4-dimensional CT (4DCT) scans, slow CT scans, a CT scan acquired at deep inspiration breath-hold or other respiratory gating strategies. 4DCT scans are widely used but may be limited by irregular breathing. While slow CT is able to produce target volumes similar to 4DCT scans, they may not accurately capture lung tumours with small respiratory movements (24,25). Deep inspiration breath hold or respiratory gating techniques minimise respiratory motion and increase normal tissue sparing from increased lung volume (26). They can be performed either involuntarily through the use of a spirometer connected to a balloon valve (Active Breathing Control, Elekta, Stockholm, Sweden) or voluntarily with visual or audio-visual biofeedback systems such as SDX (Dyn’R, Toulouse, France) or Abches (APEX Medical, Tokyo, Japan) with high reproducibility (27,28). Internal fiducials, which facilitate target verification and tumour tracking, are occasionally used but are not necessary and insertion of fiducials carries significant risks of pneumothorax in a fragile patient population (23). Accounting for inter-patient variation in respiratory motion of gross tumour volume (GTV) using individually tailored respiratory management strategies ultimately creates a patient and treatment-specific internal target volume (ITV). This represents an individualised solution, which is in sharp contrast to the early days of SBRT when patients were simulated on a single CT scan and crude population based margins were applied to account for respiratory motion. Another margin will be added to the ITV for set-up uncertainties and slight patient movements during treatment, resulting in a planning target volume (PTV). Additional margins for microscopic disease are not added during SBRT based on the understanding that any microscopic disease extending from the tumour would be dealt with by the dose fall off or penumbra (22).
Dose distribution in SBRT
In an attempt to standardize dosimetry across institutions and clinical trials, radiation therapy oncology group (RTOG) provides a set of planning guidelines to produce compact dose distributions with heterogeneous doses within the target, and steep dose gradients outside. Planning constraints limiting “hot spots” (doses greater than 105% of prescription dose) to within the PTV and enforcing a conformity index of 1.2 make the use of multiple non-opposing, non-coplanar beams with large angles between or multiple arcs with at least 180 cumulative degrees rotation imperative. At the same time, moderate dose spillage is kept to a minimum, as determined by the PTV size. Normal tissue dose-volume constraints specific to different dose-fractionations are applied. Pencil beam algorithms, which do not correct for increased electron scattering in lower-density material tend to underestimate doses in lung tumours and are not recommended in SBRT planning (21,22).
Dose-volume constraints
The determinants of dose-limiting toxicity in SBRT are organs within the thorax such as lung, central airway, bronchi, oesophagus, heart, great vessels, spinal cord and organs outside including the brachial plexus nerves, skin, stomach, small intestines, liver, chest wall and ribs. Dose-volume constraints of these normal structures are well established in conventional radiotherapy (dose per fraction of 1.8 to 2.0 Gy) and moderately hypo-fractionated schedules (dose per fraction of 3 to 5 Gy) with lower total BEDnormal tissue (29,30). While dose equivalence can be established using the LQ model, there is uncertainty when extreme hypo-fractionated (≥6 Gy) doses such as those used in SBRT are applied to small volumes, especially in serially organized tissue. Therefore, dose-volume constraints specific to different dose-fractionation schedules have been systematically defined during prospective clinical studies and correlated with observed rates of toxicities. Initially, delivering SBRT to central lung lesions was thought to be unsafe and reliable data on dose-volume constraints for mediastinal structures was not available. This has changed with a series of phase 1 dose-finding studies in central lung SBRT. The normal tissue dose-volume constraints across single-, 3-, 4-, 5- and 8-fraction lung SBRT schedules are summarised in Table 1 (21,31-37).
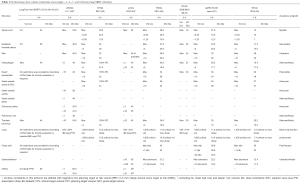
Full table
Beam delivery
While 3-dimensional conformal radiotherapy (3D-CRT) and dynamic conformal arc therapy (DCAT) offer good PTV dose distributions and adherence to normal tissue dose constraints, volumetric modulated arc therapy (VMAT) has been found to be consistently better in both regards (38,39).
The clinical significance of this dosimetric advantage is however controversial. Intriguing data from a recently published large scale retrospective analysis of 803 patients treated with SBRT across five European and North American institutions found an association between low doses to the upper regions of the heart (atria and vessels) and non-cancer deaths post SBRT. The study demonstrated that a maximum point dose to the left atrium (Dmax) of median 6.5 Gy [EQD23, α/βnormal tissue =3; hazard ratio (HR)=1.005, P=0.035] and dose to 90% of the vena cava (D90%) of median 0.59 Gy (EQD23, α/βnormal tissue =3; HR=1.025, P=0.008) were significantly associated with non-cancer deaths (40). While the link between association and causation is unclear, it is perhaps an important reminder that most patients receiving SBRT are medically inoperable with underlying cardiac and pulmonary co-morbidities. Apparently insignificant dose spillage into surrounding normal tissue may be clinically relevant and attempts should therefore be made to keep them to a minimum.
Nevertheless, beyond its dosimetric benefits, VMAT offers a shorter treatment duration and better patient comfort and compliance compared to 3D-CRT and DCAT, making it a more attractive option for SBRT (39). With VMAT or any other forms of intensity-modulated radiotherapy (IMRT) however, one has to consider the “interplay effect” and the uncertainty it brings to actual dose delivery (25). In this regard, it is perhaps reassuring that clinical outcomes from VMAT and IMRT SBRT have been excellent (39) and with measures in place such as placing constraints on multileaf collimator (MLC) motion, limiting delivery to two arcs and treatment to more than 2 fractions, the risk of clinically significant “interplay effect” in VMAT can be safely mitigated (25,41,42).
SBRT and clinical outcomes
Some of the earliest work in lung SBRT was accomplished by investigators in Japan and at the Indiana University. In a landmark study by Uematsu et al., outcomes from 50 patients treated with SBRT to dose fractionation schedules ranging from 50 to 60 Gy in 5 to 10 fractions were published in 2001. Of note, they included 18 patients who had already received prior high dose conventional radiotherapy (40–60 Gy in 20–33 fractions) and had recurred with presumably radio-resistant disease. Despite this, 47 of 50 patients achieved long-term local control (LC). Three-year overall survival (OS) was 66% and cause-specific survival was 88% (8).
Further dose escalation to improve outcomes
Meanwhile, 47 patients at Indiana University with T1-T2 N0M0 NSCLC were recruited to a dose escalation study in which they received doses starting at 8 Gy per fraction for a total of 3 fractions delivered over 2 weeks. Doses were increased in increments of 2 Gy per fraction and despite pre-existing co-morbidities, the investigators demonstrated that the maximum tolerated dose (MTD) was not reached for T1 tumours while the MTD for T2 tumours larger than 5 cm was realised at 24 Gy per fraction. Seventy-two Gy in 3 fractions was equivalent to a BED10 of 244.8 Gy, which was significantly higher than anything previously achieved through conventional fractionation without significant toxicity. Furthermore, LC was excellent with only 1 failure seen when dose per fraction was higher than 16 Gy vs. 9 failures at doses less than 16 Gy, alluding to a dose-response relationship (43,44).
This dose-response relationship became clear when a Japanese multi-institutional study led by Onishi et al. demonstrated that a minimum threshold BED10 of 100 Gy, prescribed to the isocentre, was required to achieve significantly better LC leading to improved OS (5). More recently, a large scale retrospective review of SBRT outcomes for 747 patients across 65 centres in the United States suggested this dose-response survival function continues to rise beyond the threshold BED10 of 100 Gy, extending past 105 Gy and potentially 110 Gy (45) while Koshy et al. demonstrated that for larger T2 tumours, this dose response may even continue up to a BED10 as high as 150 Gy (46). However, uncertainty remains on whether these doses were defined as PTV-encompassing doses or isocentric doses. Previous studies have suggested that isocentric doses correlate better with local TCP (17) and without knowledge of the prescription doses or dose profile, it would be difficult to draw conclusions.
To achieve further dose escalation safely, studies such as JCOG 0702 have helped guide clinical practice. In this study, the subset of peripheral T2N0M0 NSCLC (>3 cm) was specifically studied based on earlier reports demonstrating improved LC in these T2 tumours when dose was escalated from 40 to 48 Gy in 4 fractions (P=0.0015) (47). The authors concluded that for peripheral PTVs smaller than 100 cc, the MTD at the D95 of the PTV could be safely increased from 40 Gy in 4 fractions over 4–8 days (BED10 =80 Gy) to 55 Gy in 4 fractions (BED10 =130.6 Gy) (34). Larger tumours with PTV greater than 100 cc can be safely escalated to 50 Gy in 4 fractions (BED10 =112.5 Gy) (48).
Central lung SBRT
However, MTDs for peripherally located lesions cannot be applied to central lung tumours. Different definitions exists but in general, central lesions are distinguished as any tumour within or touching the zone of the proximal bronchial tree, defined as a volume of 2 cm in all directions around the proximal bronchial tree (carina, right and left main bronchi, right and left upper lobe bronchi, intermedius bronchus, right middle lobe bronchus, lingular bronchus, right and left lower lobe bronchi) as well as lesions which are immediately adjacent to the mediastinal or pericardial pleura, with a PTV expected to touch or include the pleura (6,31,49). When Timmerman et al. at the University of Indiana carried out a single-arm phase 2 study from 2002 and 2004, it quickly became obvious that SBRT to these central lung lesions resulted in higher rates of severe toxicities with 2-year freedom from severe toxicity rates of 54% (central) vs. 83% (peripheral) (P=0.004) (6) and they were subsequently excluded from the RTOG 0236 study. Other reports of severe and fatal toxicities after central lung SBRT followed (50,51). The clinical need to determine the MTDs for central lesions and establish reliable dose-volume constraints for each of the mediastinal structures resulted in investigators embarking on a series of dose-finding studies.
Dose finding studies in central lung SBRT
The EORTC sponsored LungTech trial (EORTC 22113-08113) led by Nestle et al. opened in late 2014. In this study, central tumours were treated with 60 Gy in 8 fractions and results are awaited (31). JROSG10-1 treated only T1 NSCLC patients and found the MTD to be 60 Gy in 8 fractions. At this dose, no grade 3 or worse adverse effect within 12 months of treatment was seen and all dose constraints could be met (32). RTOG 0813 led by Bezjak et al. reported their MTD to be 60 Gy in 5 fractions (1 fraction every 2 days). Thirty-three patients were treated with 60 Gy in 5 fractions with a median follow-up of 29.8 months and 38 patients were treated with 57.5 Gy in 5 fractions with a median follow-up of 33 months. Two-year LC and OS rate was upwards of 87% and 70.2% respectively. While overall rate of grade 3 or greater toxicities for all 71 patients was acceptable, the authors did report 2 (5.3%) grade 5 toxicities in the 11.5-Gy cohort and 1 (3%) grade 4 oesophageal perforation and 1 (3%) grade 5 pulmonary haemorrhage in the 12-Gy cohort (33). While these risks are low, they are severe and have to be discussed with patients. Longer fractionations appear to have a better safety profile and if efficacious, could represent a risk-adapted alternative for high-risk patients for whom severe toxicities may be catastrophic.
Single-fraction lung SBRT
The ideal dose fractionation has also been a subject of study in peripheral lung SBRT. A series of studies have looked at dose escalation in a single fraction and while many of them involve heterogeneous populations including both early stage NSCLC as well as pulmonary metastases from a variety of histologies, much can be learnt about tolerability as well as dose-response.
One of the earliest studies involving single-fraction SBRT led by Wulf et al. used 26 Gy in a single fraction to treat 1 early stage NSCLC and 25 small lung metastases including NSCLC metastases. Despite the heterogeneous disease treated, no local failures were seen with single-fraction 26 Gy at 11 months. More importantly, no severe acute or late normal tissue toxicity was observed (52).
Hof et al. treated patients with doses ranging from single-fraction 19 to 30 Gy at isocentre and found improved LC with doses equivalent to or higher than 26 Gy (P=0.032) (53). Again, no clinically significant treatment related toxicity was observed.
Meanwhile, Hara et al. treated 59 patients (11 early stage NSCLC and 48 metastases) using doses ranging from 26 Gy to more than 30 Gy (range, 30–34 Gy) and observed minimal toxicity with only 1 patient (1.7%) suffering grade 3 respiratory symptoms. Doses of 30 Gy and higher seemed to improve 2-year local progression free survival from 52% (<30 Gy) to 83% (P=0.07). However, the majority (88.1%) of tumours treated were smaller than 3 cm and maximum tumour size for all tumours was smaller than 4 cm (54). Studies have shown that larger treatment volumes greater than 50 cc (equivalent to a diameter greater than 4.5 cm) and patients who have received prior thoracic radiation are at significant risks of pulmonary toxicity even at single-fraction 25 Gy and higher doses should be used with caution (55).
RTOG 0915—fractionated vs. single-fraction SBRT
Comparing a single-fraction 34 Gy with the more commonly used 48 Gy in 4 consecutive daily fractions, RTOG 0915 aimed to ascertain the ideal lung SBRT dose fractionation. The primary end point of this study was rate of grade 3 or greater adverse events at 1 year and in this regard, single-fraction SBRT was found to be better tolerated {4 of 39 (34 Gy/1 fr) [10.3%, 95% confidence interval (CI), 2.9–24.2%] vs. 6 of 45 (48 Gy/4 fr) (13.3%, 95% CI, 5.1–26.8%)} while offering similar primary tumour control [2-year cumulative primary tumour failure rate 2.6% (34 Gy/1 fr) vs. 2.2% (48 Gy/4 fr)]. However, toxicities occurring at a later time point such as brachial plexopathies and longer-term decline in pulmonary function were not reported and could be clinically relevant. It is also important to note that while tumours less than 5 cm were eligible for the study, the median tumour diameter in the recruited single-fraction cohort was 2 cm (range, 1.00–4.98 cm) and it is possible that a higher toxicity rate may be seen if larger tumours had been treated (35).
Another concern with RTOG 0915 was that OS data beyond 1 year suggested a trend favouring 48 Gy/4 fr. This is despite the BEDtumour for single-fraction 34 Gy (BED10 =149.6 Gy) being 44 Gy higher than the BEDtumour for 48 Gy/4 fr (BED10 =105.6 Gy) (35). Even though this study was not powered to address differences in OS between the 2 treatment arms, advocates for fractionated SBRT have pointed to this to highlight concerns regarding tumour hypoxia-conferred radio-resistance, an effect thought to be more pronounced when treatment is delivered in a single fraction, therefore losing the protection that re-oxygenation offers fractionated treatments (20). Furthermore, single-fraction 34 Gy results in a much higher BEDnormal tissue (α/βnormal tissue =3) of 419.3 Gy compared to a BEDnormal tissue of 240 Gy for 48 Gy/4 fr and previous meta-analysis had found a detrimental effect on OS when BED10 exceeds 146 Gy (56), possibly due to higher dose to normal tissue resulting in increased risk of occult toxicities and non-cancer related deaths (40,57).
For these reasons, at present, fractionated lung SBRT is more widely practised. Table 2 (35,36,52-55,58-64) summarizes a series of widely referenced lung SBRT studies involving both fractionated and single-fraction regimens.

Full table
Surgery vs. SBRT
The earliest reports from Japan included a significant proportion of patients who were medically operable but refused surgery. For example, of the 257 patients reported by Onishi et al., 99 (38.5%) were medically operable. Five-year OS for these medically operable patients who received a minimum threshold BED10 of 100 Gy was 72.3% (95% CI, 59.1–85.6%) for stage IA and 65.9% (95% CI, 43.0–88.9%) for stage IB (5). This was consistent with other studies, summarised in Table 3 (5,7,8,37,65-71), which reported excellent LC and 3-year OS rates upwards of 66%. This raised the question of whether SBRT should be offered as a reasonable alternative to medically operable patients. In particular, elderly patients with small peripheral lesions and borderline lung function or medical comorbidities, for which surgery and general anaesthesia are not without risks, are thought to benefit most from non-invasive SBRT. A Dutch population-based matched-pair comparison study between SBRT and surgery for the elderly cohort found 30-day mortality to be 8.3% after surgery vs. 1.7% after SBRT while 3-year survival rates between the two modalities were similar at 60% for surgery vs. 42% for SBRT (P=0.22) (72). Across all studies, SBRT for medically operable patients was expectedly well tolerated with incidence rates of Common Terminology Criteria for Adverse Events (CTCAE) grade 3 or greater toxicity of up to 15% and a cumulative incidence of treatment related mortality of only 0.7% (68-71). On the other hand, overall complication rates from video-assisted and open thoracotomy lobectomy can be as high as 16.4% and 31.2% respectively (73) with 30-day post lobectomy mortality rates of about 2.4% (74).

Full table
Three separate randomised studies [ACOSOG Z4099 (NCT01336894), STARS (NCT00840749) and ROSEL (NCT00687986)] attempted to compare surgery with mediastinal lymph node sampling vs. SBRT delivering a minimum BED10 of 100 Gy but all suffered from poor accrual and closed prematurely. A total of 58 individual patient data from STARS and ROSEL trials were subsequently pooled and analyzed by Chang et al. Estimated OS at 3 years was 95% in the SABR group compared with 79% in the surgery group (log-rank P=0.037). The 3-year pooled estimated LC, regional control (RC) and distant control (DC) for the SBRT cohort vs. surgery cohort were similar at 96% vs. 100%, 90% vs. 96%, and 97% vs. 91%, respectively (75). SBRT appeared to be better tolerated than surgery with fewer grade 3 and greater toxicities (10% vs. 44%) and no treatment related deaths (0% vs. 4%). The authors concluded that for medically operable patients, SBRT showed at least clinical equipoise when compared to surgery. Furthermore, patient reported quality of life outcomes with SBRT have been found to be at least equal if not better than surgery (76,77).
On the other hand, with surgery and mediastinal lymph node sampling, up to 35% of patients can be upstaged, with half of these upstagings bringing about the addition of adjuvant chemotherapy (78). However, multiple studies have shown similar rates of regional and distant recurrences (62,72,75,79) for SBRT compared to surgery, perhaps due to sterilization of micrometastases through incidental mediastinal, hilar dose or the triggering of a systemic immune response against micrometastases (80). Furthermore, endobronchial lymph node sampling might be able to reduce some of the false negatives with PET-CT, which can be as high as 33.3% in higher risk central T2 lesions with a solid appearance on imaging (81,82). Isolated recurrences if they do occur can also be salvaged with definitive radiotherapy or in a few occasions salvage surgery (83-87). All in all, an argument could be made that for patients at a higher risk from surgery, SBRT should at least be discussed as an alternative.
Optimizing SBRT therapeutic ratio
Complications are rare in SBRT but relatively large hypo-fractionated doses mean that they can be potentially life threatening (33,58,88). These include central airway toxicities such as bronchial stenosis resulting in atelectasis (89), bronchial necrosis or hemoptysis (51), esophageal toxicities such as strictures, perforation or trachea-oesophageal fistulas (90), aortic toxicities such as hemoptysis secondary to aortic damage or aortic rupture, aortic aneurysm or aortic dissection (91), severe skin toxicities (92), chest wall pain including rib fractures (93-95), symptomatic radiation pneumonitis (RP) (96,97), and brachial plexopathies (98). Rarer complications include vagal nerve injury (99) and spontaneous pneumothorax (100). A post-treatment decline in pulmonary function can also be observed but it is usually not clinically significant (101).
Dosimetric parameters associated with these toxicities have been demonstrated (88-92,94,95,98,102) and have led to the establishment of dose volume constraints as previously shown in Table 1 (21,31-37). These constraints can be achieved through more precise delivery of radiotherapy with motion management strategies, intensity modulation and non-coplanar beam deliveries (38,39) backed by meticulous quality assurance. More fractionated regimens can also be used if there is a need for gentler doses to the normal tissue. This way, one can effectively kill two birds with one stone, achieving the minimum BEDtumour of 100 Gy for optimal local control and cure while reducing the toxicities of treatment by utilizing the benefits of fractionation.
However, dosimetric parameters alone do not adequately account for inter-patient variation in baseline normal tissue characteristics and intrinsic radiosensitivity. To account for these individual differences, considerations based on pre-treatment clinical, normal tissue and radiological characteristics need to be made. Pulmonary function tests are often performed prior to SBRT but appear to correlate poorly with grade ≥ 2 RP and patients with poor pulmonary function achieve cause specific survival and toxicity outcomes similar to patients with better function (103). Some institutions have sought to use baseline radiological characteristics as a means to identify patients at greater risk of RP. Subclinical interstitial lung disease manifesting as honeycombing on pre-treatment CT had been found to be associated with fatal interstitial pneumonitis post-surgery (104) and similar correlations with poor outcomes have been established in high-dose radiotherapy (66,105). Combining dosimetric parameters, age and extent of pulmonary fibrosis as determined according to the modified criteria of Kazerooni et al. (106) in a retrospective review of 122 patients, Tsujino et al. from Hyogo Cancer Centre proposed a predictive score which was able to predict the incidence of grade ≥ 3 RP with an area under the curve (AUC) of 0.888 (107). However, this cohort of patients received high dose conventional radiation concurrent with chemotherapy. The use of concurrent radiation sensitizers and likely larger treatment volumes mean that their findings may have limited applicability to SBRT treatments. Furthermore, while age is a predictive factor in this study, other studies have consistently shown that elderly patients tolerate SBRT treatments just as well as younger patients (62,72). For instance, Winship Cancer Institute of Emory University demonstrated an incidence of grade ≥3 RP of only 3.5% in their cohort of patients with a median age of 85. Interestingly, patients who were not on angiotensin converting enzyme inhibitors (ACE-I) were at a higher risk of RP [odds ratio (OR) 5.83, 95% CI, 1.29–26.32] suggesting that there are complex biological mechanisms underpinning RP (62).
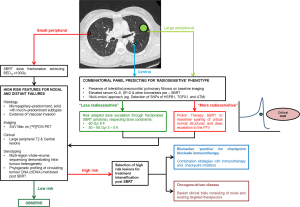
Invariably, normal tissue toxicity is the result of an acute inflammatory response within the microenvironment to radiation injury, through expression and maintenance of inflammatory cytokines, fibrotic cytokines, chemokines and recruitment of inflammatory cells, leading subsequently to scar formation or fibrosis (108,109). Identification and monitoring of blood-borne inflammatory biomarkers such as transforming growth factor β (TGFβ), IL-6, Krebs von den Lungen (KL-6) and surfactant protein (SP-D) can potentially account for inter-individual differences in responses to radiation injury beyond clinical, radiological and dosimetric parameters (109,110). Indeed, in an attempt to further stratify patients’ risk of RP, Yamashita et al. prospectively combined individual pre-treatment blood biomarkers KL-6 and SP-D with presence of interstitial pneumonitis on CT imaging. Despite all patients meeting dose constraints as per JCOG 0403 protocol, they found an increased risk of severe RP in patients with elevated KL-6 [32% (high) vs. 3% (low), P=0.0002], elevated SP-D [29% (high) vs. 3% (low), P=0.0002] and interstitial pneumonitis on CT [57% (high) vs. 2% (low), P<0.0001]. To date, these biomarkers have not been validated in a larger independent cohort prospectively but they demonstrate the clinical benefits of identifying these susceptible, more radiosensitive patients a priori (110). Furthermore, up to 80% of inter-individual differences in normal tissue toxicity can be attributed to genetic differences underpinning inflammatory and DNA damage responses (108,111,112). Discussed in greater detail in the companion review by Tan et al. (113), high throughput sequencing techniques can identify common low penetrance allelic variations predictive of a more radiosensitive phenotype. Specific to definitive thoracic radiotherapy, several studies have demonstrated associations between radiation-induced pneumonitis and single nucleotide polymorphisms (SNPs) of heat-shock protein beta-1 (HSPB1) (114), TGFβ1 (115), ataxia-telangiectasia mutated (ATM) and Nijmegen breakage syndrome 1 (NBS1) (116) genes but they have not been validated in large-scale prospective genome-wide association studies (GWAS). Nevertheless, these genotyping findings, when integrated with epigenetic factors, post-translational modifications, cell signalling networks and metabolism in an all-encompassing “omics” approach, allow identification of critical pathways and complex interactions crucial to the development of normal tissue toxicity (112). Combined with imaging features, blood-borne biomarkers and functional cellular assays, this integrated predictive model can add a paradigm of biological precision to risk stratification and dose prescription in high risk SBRT involving central tumours or larger (≥ T2) peripheral tumours with borderline normal tissue doses; patients predicted to be at risk of toxicities can either be offered a more conservative dose regimen or alternative means of dose escalation can be explored by exploiting the dosimetric advantages of particle therapy, a technology which will be discussed in greater detail shortly. On the other hand, patients at low risk of toxicities can have their dose escalated further to maximise TCP. This risk-stratified approach is illustrated in Figure 1.
Future directions
Moving forward, exciting developments on the horizon offer new strategies and technologies, which can be complementary to and synergistic with lung SBRT. Particle beam therapy (PBT) enables irradiation of tumours at depth while allowing a very sharp dose gradient distal to the target. Initially limited to large teaching institutions, recent innovations have helped to drive down the size, cost and complexity of PBT facilities, making them far more accessible. Examples of these innovations include the vertically arranged proton therapy system in Aizawa Hospital, Matsumoto, Japan (Sumitomo Heavy Industries, Tokyo, Japan), the S250 (Mevion, Massachusetts, US) with its gantry mounted proton accelerator and superconducting synchrocyclotron and the Radiance 330 (ProTom International, Texas, US) with its modular design allowing customisation based on individual facility needs. On the back of these innovations, the number of PBT centres is expected to rise and it is projected that by 2017, there would be 27 PBT centres in the United States (US) alone (117). While concerns surrounding organ motion and interplay effects in lung PBT remain (118), with further improvements in motion management technology, the superior dosimetry offered by PBT can be harnessed to enhance tumour dose escalation and critical tissue sparing, especially when treating central lung tumours. In addition, particle irradiation may stimulate changes within the tumour microenvironment, which could potentially suppress metastatic processes such as cell migration and invasion (119). So far, early comparisons suggest that hypo-fractionation through PBT may offer additional clinical benefits over SBRT with conventional X-rays. In a systematic review by Chi et al. which pooled 72 SBRT studies and 9 single-arm hypo-fractionated PBT studies from 2000 to 2016, before adjusting for potential confounding variables, PBT was associated with improved OS (P=0.005) and PFS (P=0.01) while significantly reducing rates of ≥ grade 3 RP (P<0.001), ≥ grade 3 chest-wall toxicities (P=0.03) and rib fractures (P<0.001) (120). As PBT technology continues to evolve, direct comparison studies will be eagerly awaited.
In recent times, immune modulating strategies have dominated the headlines in oncology. The fervent embrace of immunotherapy in NSCLC started with early successes using checkpoint inhibitors in metastatic disease (121-123). In the companion review by Tharmalingam and Hoskin, the authors concluded that SBRT is most systematically immunogenic as demonstrated in both in vivo murine models (15,124) and reported clinical cases (125) and would therefore be the ideal candidate for combination with immune modulating strategies (126). This approach will be the subject of investigation in the Dutch PEMBRO-RT study (127) (NCT02492568) but only in the setting of metastatic disease. In early stage disease, nodal and distant failures can be as high as 30% (36,60). Histologic features such as micropapillary-predominant, solid with mucin-predominant subtypes (128,129) and vascular invasion (130), pre-treatment SUV max on 18F-FDG PET (131) and gene-expression profiles (130,132) can predict for higher risk early stage disease (133) and stratify these patients for treatment intensification with further systemic treatment or combination with immunotherapy. To this end, large-scale genotyping efforts such as the Lung TRACERx Study (134) have provided new insights into tumour evolution and biology. Multi-region whole-exome sequencing of 100 early stage NSCLC in the TRACERx cohort found that intra-tumour heterogeneity, mediated through chromosome instability, was associated with an increased risk of recurrence or death following surgery (HR=4.9, P=4.4×10−4) (135). Furthermore, owing to the sub-clonal nature of early stage lung cancer relapse and metastasis, tumour-specific phylogenetic profiling of circulating tumour DNA (ctDNA) in serially collected liquid biopsies, was able to quantify sustained presence of sub-clonal single nucleotide variants in ctDNA post-surgery, which preceded subsequent relapse and adjuvant chemotherapy resistance (136). Taken together, high throughput genomic profiling of tumours and ctDNA can be exploited to identify “high risk” early stage NSCLC for treatment intensification following SBRT. This strategy is illustrated in Figure 1.
Conclusions
In the past 2 decades, through a series of well-planned clinical trials and advancements in radiotherapy technology, lung SBRT has cemented its place in the management of early stage NSCLC. The era of precision medicine has not only divulged new insights into the biology of this disease but also into inter-individual differences in responses to radiation injury. Going forward, with new tools at our disposal, these insights will inspire new intelligent strategies to further improve our cure rates of early stage NSCLC.
Acknowledgements
Funding: ML Chua is supported by the National Medical Research Council Singapore Transition Award (#NMRC/TA/0030/2014), and the Duke-NUS Oncology Academic Program Proton Research Program.
Footnote
Conflicts of Interest: KL Chua has received speaker honorarium from Varian Medical Systems; ML Chua has received speaker honorarium from Varian Medical Systems, and research funding from GenomeDx Biosciences. The remaining authors have no conflicts of interest to declare.
References
- Perez CA, Bauer M, Edelstein S, et al. Impact of tumor control on survival in carcinoma of the lung treated with irradiation. Int J Radiat Oncol Biol Phys 1986;12:539-47. [Crossref] [PubMed]
- Mehta N, King CR, Agazaryan N, et al. Stereotactic body radiation therapy and 3-dimensional conformal radiotherapy for stage I non-small cell lung cancer: A pooled analysis of biological equivalent dose and local control. Pract Radiat Oncol 2012;2:288-95. [Crossref] [PubMed]
- Saunders M, Dische S, Barrett A, et al. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: mature data from the randomised multicentre trial. CHART Steering committee. Radiother Oncol 1999;52:137-48. [Crossref] [PubMed]
- Nyman J, Hallqvist A, Lund JA, et al. SPACE - A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol 2016;121:1-8. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005;63:1427-31. [Crossref] [PubMed]
- Uematsu M, Shioda A, Suda A, et al. Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer: a 5-year experience. Int J Radiat Oncol Biol Phys 2001;51:666-70. [Crossref] [PubMed]
- Lo SS, Fakiris AJ, Chang EL, et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol 2010;7:44-54. [Crossref] [PubMed]
- Ohri N, Werner-Wasik M, Grills IS, et al. Modeling local control after hypofractionated stereotactic body radiation therapy for stage I non-small cell lung cancer: a report from the elekta collaborative lung research group. Int J Radiat Oncol Biol Phys 2012;84:e379-84. [Crossref] [PubMed]
- Iwata H, Shibamoto Y, Murata R, et al. Estimation of errors associated with use of linear-quadratic formalism for evaluation of biologic equivalence between single and hypofractionated radiation doses: an in vitro study. Int J Radiat Oncol Biol Phys 2009;75:482-8. [Crossref] [PubMed]
- Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003;300:1155-9. [Crossref] [PubMed]
- Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell 2005;8:89-91. [Crossref] [PubMed]
- Park HJ, Griffin RJ, Hui S, et al. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res 2012;177:311-27. [Crossref] [PubMed]
- Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005;174:7516-23. [Crossref] [PubMed]
- Roberts SA, Hendry JH. A realistic closed-form radiobiological model of clinical tumor-control data incorporating intertumor heterogeneity. Int J Radiat Oncol Biol Phys 1998;41:689-99. [Crossref] [PubMed]
- Guckenberger M, Klement RJ, Allgäuer M, et al. Applicability of the linear-quadratic formalism for modeling local tumor control probability in high dose per fraction stereotactic body radiotherapy for early stage non-small cell lung cancer. Radiother Oncol 2013;109:13-20. [Crossref] [PubMed]
- Shuryak I, Carlson DJ, Brown JM, et al. High-dose and fractionation effects in stereotactic radiation therapy: Analysis of tumor control data from 2965 patients. Radiother Oncol 2015;115:327-34. [Crossref] [PubMed]
- Brown JM, Brenner DJ, Carlson DJ. Dose escalation, not "new biology," can account for the efficacy of stereotactic body radiation therapy with non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;85:1159-60. [Crossref] [PubMed]
- Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys 2014;88:254-62. [Crossref] [PubMed]
- Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 2010;37:4078-101. [Crossref] [PubMed]
- Timmerman R, Galvin J, Michalski J, et al. Accreditation and quality assurance for Radiation Therapy Oncology Group: Multicenter clinical trials using Stereotactic Body Radiation Therapy in lung cancer. Acta Oncol 2006;45:779-86. [Crossref] [PubMed]
- Slotman BJ. Stereotactic body radiotherapy for stage I non-small cell lung cancer. J Radiosurg SBRT 2011;1:63-9.
- Nakamura M, Narita Y, Matsuo Y, et al. Geometrical differences in target volumes between slow CT and 4D CT imaging in stereotactic body radiotherapy for lung tumors in the upper and middle lobe. Med Phys 2008;35:4142-8. [Crossref] [PubMed]
- Yamashita H, Takahashi W, Haga A, et al. Stereotactic body radiotherapy for small lung tumors in the University of Tokyo Hospital. Biomed Res Int 2014;2014:136513.
- Mageras GS, Yorke E. Deep inspiration breath hold and respiratory gating strategies for reducing organ motion in radiation treatment. Semin Radiat Oncol 2004;14:65-75. [Crossref] [PubMed]
- Onishi H, Kawakami H, Marino K, et al. A simple respiratory indicator for irradiation during voluntary breath holding: a one-touch device without electronic materials. Radiology 2010;255:917-23. [Crossref] [PubMed]
- Giraud P, Houle A. Respiratory Gating for Radiotherapy: Main Technical Aspects and Clinical Benefits. ISRN Pulmonol. 2013;2013:13.
- Kwa SL, Lebesque JV, Theuws JC, et al. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys 1998;42:1-9. [Crossref] [PubMed]
- Cheung PC, Yeung LT, Basrur V, et al. Accelerated hypofractionation for early-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2002;54:1014-23. [Crossref] [PubMed]
- Adebahr S, Collette S, Shash E, et al. LungTech, an EORTC Phase II trial of stereotactic body radiotherapy for centrally located lung tumours: a clinical perspective. Br J Radiol 2015;88:20150036. [Crossref] [PubMed]
- Kimura T, Nagata Y, Harada H, et al. Phase I study of stereotactic body radiation therapy for centrally located stage IA non-small cell lung cancer (JROSG10-1). Int J Clin Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Bezjak A, Paulus R, Gaspar LE, et al. Efficacy and Toxicity Analysis of NRG Oncology/RTOG 0813 Trial of Stereotactic Body Radiation Therapy (SBRT) for Centrally Located Non-Small Cell Lung Cancer (NSCLC). Int J Radiat Oncol Biol Phys 2016;96:S8. [Crossref]
- Onimaru R, Shirato H, Shibata T, et al. Phase I study of stereotactic body radiation therapy for peripheral T2N0M0 non-small cell lung cancer with PTV<100 cc using a continual reassessment method (JCOG0702). Radiother Oncol 2015;116:276-80. [Crossref] [PubMed]
- Videtic GM, Hu C, Singh AK, et al. A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer: NRG Oncology RTOG 0915 (NCCTG N0927). Int J Radiat Oncol Biol Phys 2015;93:757-64. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Timmerman RD, Paulus R, Pass HI, et al. RTOG 0618: Stereotactic body radiation therapy (SBRT) to treat operable early-stage lung cancer patients. J Clin Oncol 2013;31:7523.
- Rauschenbach BM, Mackowiak L, Malhotra HK. A dosimetric comparison of three-dimensional conformal radiotherapy, volumetric-modulated arc therapy, and dynamic conformal arc therapy in the treatment of non-small cell lung cancer using stereotactic body radiotherapy. J Appl Clin Med Phys 2014;15:4898. [Crossref] [PubMed]
- Sapkaroski D, Osborne C, Knight KA. A review of stereotactic body radiotherapy - is volumetric modulated arc therapy the answer? J Med Radiat Sci 2015;62:142-51. [Crossref] [PubMed]
- Stam B, Peulen H, Guckenberger M, et al. Dose to heart substructures is associated with non-cancer death after SBRT in stage I-II NSCLC patients. Radiother Oncol 2017;123:370-5. [Crossref] [PubMed]
- Ong CL, Dahele M, Slotman BJ, et al. Dosimetric impact of the interplay effect during stereotactic lung radiation therapy delivery using flattening filter-free beams and volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys 2013;86:743-8. [Crossref] [PubMed]
- Verbakel WF, Senan S, Cuijpers JP, et al. Rapid delivery of stereotactic radiotherapy for peripheral lung tumors using volumetric intensity-modulated arcs. Radiother Oncol 2009;93:122-4. [Crossref] [PubMed]
- Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003;124:1946-55. [Crossref] [PubMed]
- McGarry RC, Papiez L, Williams M, et al. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: phase I study. Int J Radiat Oncol Biol Phys 2005;63:1010-5. [Crossref] [PubMed]
- Stahl JM, Ross R, Harder EM, et al. The Effect of Biologically Effective Dose and Radiation Treatment Schedule on Overall Survival in Stage I Non-Small Cell Lung Cancer Patients Treated With Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys 2016;96:1011-20. [Crossref] [PubMed]
- Koshy M, Malik R, Weichselbaum RR, et al. Increasing radiation therapy dose is associated with improved survival in patients undergoing stereotactic body radiation therapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2015;91:344-50. [Crossref] [PubMed]
- Onimaru R, Fujino M, Yamazaki K, et al. Steep dose-response relationship for stage I non-small-cell lung cancer using hypofractionated high-dose irradiation by real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:374-81. [Crossref] [PubMed]
- Onimaru R, Onishi H, Shibata T, et al. Phase I study of stereotactic body radiation therapy for peripheral T2N0M0 non-small cell lung cancer (JCOG0702): Results for the group with PTV 100cc. Radiother Oncol 2017;122:281-5. [Crossref] [PubMed]
- Haasbeek CJ, Lagerwaard FJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011;6:2036-43. [Crossref] [PubMed]
- Nishimura S, Takeda A, Sanuki N, et al. Toxicities of organs at risk in the mediastinal and hilar regions following stereotactic body radiotherapy for centrally located lung tumors. J Thorac Oncol 2014;9:1370-6. [Crossref] [PubMed]
- Corradetti MN, Haas AR, Rengan R. Central-airway necrosis after stereotactic body-radiation therapy. N Engl J Med 2012;366:2327-9. [Crossref] [PubMed]
- Wulf J, Haedinger U, Oppitz U, et al. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: a noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys 2004;60:186-96. [Crossref] [PubMed]
- Hof H, Muenter M, Oetzel D, et al. Stereotactic single-dose radiotherapy (radiosurgery) of early stage nonsmall-cell lung cancer (NSCLC). Cancer 2007;110:148-55. [Crossref] [PubMed]
- Hara R, Itami J, Kondo T, et al. Clinical outcomes of single-fraction stereotactic radiation therapy of lung tumors. Cancer 2006;106:1347-52. [Crossref] [PubMed]
- Le QT, Loo BW, Ho A, et al. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. J Thorac Oncol 2006;1:802-9. [Crossref] [PubMed]
- Zhang J, Yang F, Li B, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for Stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys 2011;81:e305-16. [Crossref] [PubMed]
- Guckenberger M. Dose and Fractionation in Stereotactic Body Radiation Therapy for Stage I Non-Small Cell Lung Cancer: Lessons Learned and Where Do We Go Next? Int J Radiat Oncol Biol Phys 2015;93:765-8. [Crossref] [PubMed]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677-82. [Crossref] [PubMed]
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. [Crossref] [PubMed]
- Timmerman RD, Hu C, Michalski J, et al. Long-term Results of RTOG 0236: A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Medically Inoperable Stage I Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2014;90:S30. [Crossref]
- Videtic GM, Stephans K, Reddy C, et al. Intensity-modulated radiotherapy-based stereotactic body radiotherapy for medically inoperable early-stage lung cancer: excellent local control. Int J Radiat Oncol Biol Phys 2010;77:344-9. [Crossref] [PubMed]
- Cassidy RJ, Patel PR, Zhang X, et al. Stereotactic Body Radiotherapy for Early-stage Non-small-cell Lung Cancer in Patients 80 Years and Older: A Multi-center Analysis. Clin Lung Cancer 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer 2010;68:72-7. [Crossref] [PubMed]
- Fritz P, Kraus HJ, Mühlnickel W. el al. Stereotactic, single-dose irradiation of stage I non-small cell lung cancer and lung metastases. Radiat Oncol 2006;1:30. [Crossref] [PubMed]
- Koto M, Takai Y, Ogawa Y, et al. A phase II study on stereotactic body radiotherapy for stage I non-small cell lung cancer. Radiother Oncol 2007;85:429-34. [Crossref] [PubMed]
- Onishi H, Yoshiyuki S, Yasuo M, et al. Japanese Multi-institutional Study of Stereotactic Body Radiation Therapy for More Than 2000 Patients With Stage I Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2013;87:S9-S10. [Crossref]
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989-96. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [Crossref] [PubMed]
- Eriguchi T, Takeda A, Sanuki N, et al. Stereotactic body radiotherapy for operable early-stage non-small cell lung cancer. Lung Cancer 2017;109:62-7. [Crossref] [PubMed]
- Onishi H, Shioyama Y, Matsumoto Y, et al. Japanese Multi-institutional Study of Stereotactic Body Radiation Therapy for 661 Medically Operable Patients With Stage I Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2015;93:S187. [Crossref]
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:348-53. [Crossref] [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011;101:240-4. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- Pezzi CM, Mallin K, Mendez AS, et al. Ninety-day mortality after resection for lung cancer is nearly double 30-day mortality. J Thorac Cardiovasc Surg 2014;148:2269-77. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Lagerwaard FJ, Aaronson NK, Gundy CM, et al. Patient-reported quality of life after stereotactic ablative radiotherapy for early-stage lung cancer. J Thorac Oncol 2012;7:1148-54. [Crossref] [PubMed]
- Louie AV, van Werkhoven E, Chen H, et al. Patient reported outcomes following stereotactic ablative radiotherapy or surgery for stage IA non-small-cell lung cancer: Results from the ROSEL multicenter randomized trial. Radiother Oncol 2015;117:44-8. [Crossref] [PubMed]
- Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:377-86. [Crossref] [PubMed]
- Wink KCJ, van Baardwijk A, Troost EGC, et al. Nodal recurrence after stereotactic body radiotherapy for early stage non-small cell lung cancer: Incidence and proposed risk factors. Cancer Treat Rev 2017;56:8-15. [Crossref] [PubMed]
- Bernstein MB, Krishnan S, Hodge JW, et al. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol 2016;13:516-24. [Crossref] [PubMed]
- Tournoy KG, Keller SM, Annema JT. Mediastinal staging of lung cancer: novel concepts. Lancet Oncol 2012;13:e221-9. [Crossref] [PubMed]
- Gao SJ, Kim AW, Puchalski JT, et al. Indications for invasive mediastinal staging in patients with early non-small cell lung cancer staged with PET-CT. Lung Cancer 2017;109:36-41. [Crossref] [PubMed]
- Amendola BE, Amendola MA, Perez N, et al. Local failure after primary radiotherapy in lung cancer: Is there a role for SBRT? Rep Pract Oncol Radiother 2015;20:440-5. [Crossref] [PubMed]
- Hamaji M, Chen F, Matsuo Y, et al. Treatment and Prognosis of Isolated Local Relapse after Stereotactic Body Radiotherapy for Clinical Stage I Non-Small-Cell Lung Cancer: Importance of Salvage Surgery. J Thorac Oncol 2015;10:1616-24. [Crossref] [PubMed]
- Nishimura S, Takeda A, Sanuki N, et al. Dose-Escalated Stereotactic Body Radiotherapy (SBRT) as a Salvage Treatment for Two Cases with Relapsed Peripheral Lung Cancer After Initial SBRT. J Thorac Oncol 2015;10:e69-71. [Crossref] [PubMed]
- Hearn JW, Videtic GM, Djemil T, et al. Salvage stereotactic body radiation therapy (SBRT) for local failure after primary lung SBRT. Int J Radiat Oncol Biol Phys 2014;90:402-6. [Crossref] [PubMed]
- Neri S, Takahashi Y, Terashi T, et al. Surgical treatment of local recurrence after stereotactic body radiotherapy for primary and metastatic lung cancers. J Thorac Oncol 2010;5:2003-7. [Crossref] [PubMed]
- Kang KH, Okoye CC, Patel RB, et al. Complications from Stereotactic Body Radiotherapy for Lung Cancer. Cancers (Basel) 2015;7:981-1004. [Crossref] [PubMed]
- Karlsson K, Nyman J, Baumann P, et al. Retrospective cohort study of bronchial doses and radiation-induced atelectasis after stereotactic body radiation therapy of lung tumors located close to the bronchial tree. Int J Radiat Oncol Biol Phys 2013;87:590-5. [Crossref] [PubMed]
- Abelson JA, Murphy JD, Loo BW Jr, et al. Esophageal tolerance to high-dose stereotactic ablative radiotherapy. Dis Esophagus 2012;25:623-9. [Crossref] [PubMed]
- Evans JD, Gomez DR, Amini A, et al. Aortic dose constraints when reirradiating thoracic tumors. Radiother Oncol 2013;106:327-32. [Crossref] [PubMed]
- Hoppe BS, Laser B, Kowalski AV, et al. Acute skin toxicity following stereotactic body radiation therapy for stage I non-small-cell lung cancer: who's at risk? Int J Radiat Oncol Biol Phys 2008;72:1283-6. [Crossref] [PubMed]
- Nambu A, Onishi H, Aoki S, et al. Rib fracture after stereotactic radiotherapy on follow-up thin-section computed tomography in 177 primary lung cancer patients. Radiat Oncol 2011;6:137. [Crossref] [PubMed]
- Stam B, van der Bijl E, Peulen H, et al. Dose-effect analysis of radiation induced rib fractures after thoracic SBRT. Radiother Oncol 2017;123:176-81. [Crossref] [PubMed]
- Andolino DL, Forquer JA, Henderson MA, et al. Chest wall toxicity after stereotactic body radiotherapy for malignant lesions of the lung and liver. Int J Radiat Oncol Biol Phys 2011;80:692-7. [Crossref] [PubMed]
- Seppenwoolde Y, De Jaeger K, Boersma LJ, et al. Regional differences in lung radiosensitivity after radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;60:748-58. [Crossref] [PubMed]
- Tucker SL, Jin H, Wei X, et al. Impact of toxicity grade and scoring system on the relationship between mean lung dose and risk of radiation pneumonitis in a large cohort of patients with non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2010;77:691-8. [Crossref] [PubMed]
- Forquer JA, Fakiris AJ, Timmerman RD, et al. Brachial plexopathy from stereotactic body radiotherapy in early-stage NSCLC: dose-limiting toxicity in apical tumor sites. Radiother Oncol 2009;93:408-13. [Crossref] [PubMed]
- Shultz DB, Trakul N, Maxim PG, et al. Vagal and recurrent laryngeal neuropathy following stereotactic ablative radiation therapy in the chest. Pract Radiat Oncol 2014;4:272-8. [Crossref] [PubMed]
- Ohnishi K, Shioyama Y, Nomoto S, et al. Spontaneous pneumothorax after stereotactic radiotherapy for non-small-cell lung cancer. Jpn J Radiol 2009;27:269-74. [Crossref] [PubMed]
- Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function Test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol 2009;4:838-44. [Crossref] [PubMed]
- Guckenberger M, Baier K, Polat B, et al. Dose-response relationship for radiation-induced pneumonitis after pulmonary stereotactic body radiotherapy. Radiother Oncol 2010;97:65-70. [Crossref] [PubMed]
- Guckenberger M, Kestin LL, Hope AJ, et al. Is there a lower limit of pretreatment pulmonary function for safe and effective stereotactic body radiotherapy for early-stage non-small cell lung cancer? J Thorac Oncol 2012;7:542-51. [Crossref] [PubMed]
- Ito H, Nakayama H, Tsuboi M, et al. Subpleural honeycombing on high resolution computed tomography is risk factor for fatal pneumonitis. Ann Thorac Surg 2011;91:874-8. [Crossref] [PubMed]
- Sanuki N, Ono A, Komatsu E, et al. Association of computed tomography-detected pulmonary interstitial changes with severe radiation pneumonitis for patients treated with thoracic radiotherapy. J Radiat Res 2012;53:110-6. [Crossref] [PubMed]
- Kazerooni EA, Martinez FJ, Flint A, et al. Thin-section CT obtained at 10-mm increments versus limited three-level thin-section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. AJR Am J Roentgenol 1997;169:977-83. [Crossref] [PubMed]
- Tsujino K, Hashimoto T, Shimada T, et al. Combined analysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves prediction of severe radiation pneumonitis after concurrent chemoradiotherapy for locally advanced non-small-cell lung cancer. J Thorac Oncol 2014;9:983-90. [Crossref] [PubMed]
- Barnett GC, West CM, Dunning AM, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer 2009;9:134-42. [Crossref] [PubMed]
- Kong FM, Ao X, Wang L, et al. The use of blood biomarkers to predict radiation lung toxicity: a potential strategy to individualize thoracic radiation therapy. Cancer Control 2008;15:140-50. [Crossref] [PubMed]
- Yamashita H, Kobayashi-Shibata S, Terahara A, et al. Prescreening based on the presence of CT-scan abnormalities and biomarkers (KL-6 and SP-D) may reduce severe radiation pneumonitis after stereotactic radiotherapy. Radiat Oncol 2010;5:32. [Crossref] [PubMed]
- Turesson I, Nyman J, Holmberg E, et al. Prognostic factors for acute and late skin reactions in radiotherapy patients. Int J Radiat Oncol Biol Phys 1996;36:1065-75. [Crossref] [PubMed]
- Herskind C, Talbot CJ, Kerns SL, et al. Radiogenomics: A systems biology approach to understanding genetic risk factors for radiotherapy toxicity? Cancer Lett 2016;382:95-109. [Crossref] [PubMed]
- Tan JS, Liu X, Chua KL. Exploiting molecular genomics in precision radiation oncology: a marriage of biological and physical precision. Chin Clin Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Pang Q, Wei Q, Xu T, et al. Functional promoter variant rs2868371 of HSPB1 is associated with risk of radiation pneumonitis after chemoradiation for non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;85:1332-9. [Crossref] [PubMed]
- Yuan X, Liao Z, Liu Z, et al. Single nucleotide polymorphism at rs1982073:T869C of the TGFbeta 1 gene is associated with the risk of radiation pneumonitis in patients with non-small-cell lung cancer treated with definitive radiotherapy. J Clin Oncol 2009;27:3370-8. [Crossref] [PubMed]
- Zhang L, Wang L, Yang M, et al. Association of DNA Repair and Inflammatory Cytokines Gene Polymorphisms With Radiation-Induced Toxicity in Lung Cancer. Int J Radiat Oncol Biol Phys 2007;69:S60-1. [Crossref]
- 27 U.S. Proton Therapy Centers Expected by 2017. Available online: https://www.itnonline.com/article/27-us-proton-therapy-centers-expected-2017
- De Ruysscher D, Sterpin E, Haustermans K, et al. Tumour Movement in Proton Therapy: Solutions and Remaining Questions: A Review. Cancers (Basel) 2015;7:1143-53. [Crossref] [PubMed]
- Ogata T, Teshima T, Kagawa K, et al. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res 2005;65:113-20. [PubMed]
- Chi A, Chen H, Wen S, et al. Comparison of particle beam therapy and stereotactic body radiotherapy for early stage non-small cell lung cancer: A systematic review and hypothesis-generating meta-analysis. Radiother Oncol 2017;123:346-54. [Crossref] [PubMed]
- Dempke WC, Fenchel K. Pembrolizumab as first-line treatment for non-small cell lung cancer-a game changer? Transl Lung Cancer Res 2016;5:538-42. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Morgensztern D, Herbst RS. Nivolumab and Pembrolizumab for Non-Small Cell Lung Cancer. Clin Cancer Res 2016;22:3713-7. [Crossref] [PubMed]
- Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15:5379-88. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Tharmalingam H, Hoskin PJ. The optimism surrounding stereotactic body radiation therapy and immunomodulation. Chin Clin Oncol 2017;6:S9. [Crossref] [PubMed]
- Pembrolizumab After SBRT Versus Pembrolizumab Alone in Advanced NSCLC (PEMBRO-RT). Available online: https://clinicaltrials.gov/ct2/show/NCT02492568
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [Crossref] [PubMed]
- Leeman JE, Rimner A, Montecalvo J, et al. Histologic Subtype in Core Lung Biopsies of Early-Stage Lung Adenocarcinoma is a Prognostic Factor for Treatment Response and Failure Patterns After Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys 2017;97:138-45. [Crossref] [PubMed]
- Harpole DH Jr, Herndon JE 2nd, Wolfe WG, et al. A prognostic model of recurrence and death in stage I non-small cell lung cancer utilizing presentation, histopathology, and oncoprotein expression. Cancer Res 1995;55:51-6. [PubMed]
- Kohutek ZA, Wu AJ, Zhang Z, et al. FDG-PET maximum standardized uptake value is prognostic for recurrence and survival after stereotactic body radiotherapy for non-small cell lung cancer. Lung Cancer 2015;89:115-20. [Crossref] [PubMed]
- Beer DG, Kardia SL, Huang CC, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med 2002;8:816-24. [PubMed]
- Taunk NK, Rimner A, Culligan M, et al. Immunotherapy and radiation therapy for operable early stage and locally advanced non-small cell lung cancer. Transl Lung Cancer Res 2017;6:178-85. [Crossref] [PubMed]
- Jamal-Hanjani M, Hackshaw A, Ngai Y, et al. Tracking genomic cancer evolution for precision medicine: the lung TRACERx study. PLoS Biol 2014;12:e1001906. [Crossref] [PubMed]
- Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the Evolution of Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2109-21. [Crossref] [PubMed]
- Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017;545:446-51. [Crossref] [PubMed]


