Stereotactic body radiotherapy for primary renal cell carcinoma and adrenal metastases
Why stereotactic body radiotherapy (SBRT) for primary renal cell carcinoma (RCC) and adrenal metastases?
The increasing availability and access to cross-sectional diagnostic imaging has led to a rising incidence of both RCC and isolated adrenal metastases. Both these disease states present therapeutic challenges due to several factors, including an ageing patient population, patient co-morbidities, and the complexities of managing oligometastatic disease (1-4). Historically both RCC and oligometastatic adrenal lesions have been managed surgically with good effect. There are however several situations in which surgery is not ideal, such as in medically inoperable patients, patients with bilateral renal tumors or single kidneys, and in patients with pre-existing chronic renal failure (CRF). In the oligometastatic setting, surgery represents an invasive management option that in some cases pose unacceptable risks to the patient. Some disease, for example larger or locally invasive adrenal gland metastases may be technically challenging to completely resect without considerable associated morbidity. Therefore less invasive options are increasingly considered including radiofrequency ablation (RFA), cryotherapy, microwave ablation and more recently, SBRT. Advantages of SBRT include its ability to provide a non-invasive ablative option, the capacity to treat larger tumors, and evidence suggestive of excellent local control (LC) rates and low rates of toxicity. This review will focus on the use of SBRT for primary RCC, adrenal metastases, and oligometastatic RCC. Given the anatomical proximity of the adrenal gland and the kidney, it is pertinent to consider in conjunction the technical issues involved with delivery of SBRT to these sites. This review will highlight both the challenges and opportunities SBRT presents, in terms of its indications, technical considerations, clinical outcomes and safety data within these tumor contexts. It will conclude by discussing a few special considerations and future directions. It is hoped that this review may provide guidance to both established and developing SBRT practitioners and centers.
Emerging indications for SBRT
Although nephrectomy remains the standard of care treatment for primary RCC, there are several situations where SBRT may present an alternative solution. The potential indications for SBRT in primary RCC include medically unfit patients, bilateral kidney malignancies, patients with CRF, and patients with a single functioning kidney. SBRT, does however, present unique technical challenges, particularly in patients in whom the target volume has proximity to the bowel and in patients with very large tumors (5), although data is lacking as to the size limit at which SBRT is no longer considered a viable or effective option (6). While probe-based ablative techniques for RCC treatment exist, including RFA and cryotherapy, compared to these, SBRT is usually less invasive, and often more suitable for frail patients including those on anticoagulation. SBRT is able to treat more centrally placed tumors, larger (>4 cm) tumors (7,8), and tumors situated adjacent to the collecting system and vessels, without the fistula and stricture concerns in these cases seen with other modalities. For small renal masses, a meta-analysis suggests that cryotherapy has a small but significant advantage over RFA in terms of LC (94.8% versus 87.1%) (9), albeit with a greater proportion of laparoscopic procedures in the cryotherapy literature as compared to mostly percutaneous approaches with RFA. Whilst retrospective studies suggest for smaller tumors that SBRT may have comparable results to thermal ablation, there is no randomized data available, and unfortunately, the first randomized trial opened comparing SBRT and RFA has recently been closed early due to lack of funding (Clinical trials database: NCT02138578). For larger tumors, there is data to support an increased complication rate in patients treated with cryotherapy, and for this group of patients, SBRT may be an advantageous alternative option. A prospective analysis of 99 patients with cT1 RCC treated with cryotherapy found the risk of intra-operative complications on multivariate analysis to be significantly correlated to tumor diameter, surface and volume, with a diameter of 35 mm being predictive for an increased risk of complications (10). Another study retrospectively identified 124 patients who underwent cryotherapy at a single institution and found the risk of disease progression and cryotherapy failure to be significantly associated with hilar disease location and tumor size greater than 3 cm (11). Whilst thermal ablative techniques are highly dependent on operator experience, SBRT can be protocolized and is permissive of standardized dosimetric planning, quality assurance and peer review of plans, translating to a high level of reproducibility of outcomes and a less steep ‘learning curve’.
Metastatic disease at presentation and the development of metastases in RCC is not uncommon (12). Increasingly patients are being diagnosed with oligometastatic disease, a term initially introduced in 1995 to represent patients in an intermediary state between localized and widely metastatic disease, in which long term disease free survival or even cure may be possible (13). Cytoreductive nephrectomy (14) and metastasectomy (15) historically have been standard management options for patients with good prognosis metastatic RCC. More recently however the role of surgery to the primary in the context of targeted therapies is being questioned through a number of clinical trials (Clinical trials database: NCT02535351, NCT00930033, NCT01099423). SBRT provides a less invasive option in this group of patients with non-randomized data showing excellent rates of LC with minimal toxicities (16), making it an attractive but as yet untested approach. Future studies addressing the role of SBRT to the primary tumor as an alternative to cytoreductive nephrectomy in the context of established metastatic disease are required.
Similarly, patients presenting with adrenal oligometastatic disease are often treated with surgery as a method of obtaining LC, with radiotherapy reserved for palliation of symptoms using conventional methods (17,18). There are studies supporting long-term survival following aggressive treatment of these patients (19-22). SBRT in oligometastatic adrenal disease may provide a non-invasive option that may have the added benefit of improved hormonal function preservation (23). It may also be preferable to adrenalectomy in cases with involvement of the adrenal capsule or vascular pedicle. Hormonal deficits after stereotactic radiotherapy are gradual in onset and expected to manifest typically years after treatment (23,24), whilst after adrenalectomy hormonal dysfunction is abrupt and profound, particularly in patients with a solitary functioning adrenal gland. Since 2013, SBRT has been listed as an alternative option to surgery by the NCCN guidelines for adrenal tumors (25).
RCC, immunomodulation, and the abscopal effect
An abscopal effect is a rare effect which occurs “at a distance from the irradiated volume but within the same organism,” (26). A systematic review on abscopal effects found seven case reports of abscopal effects in patients with RCC, with other common primaries being melanoma and lymphoma (27,28). The mechanism of action of the abscopal effect is poorly understood. There are several hypothesized mechanisms with an immunological basis, with triggers that include stereotactic radiotherapy, although our ability to predict or harness this phenomenon still remains to be seen (29,30). In recent times, immunomodulatory agents have revolutionized the treatment of metastatic disease. Historically, interleukin-2 and more recently PD-1 inhibitors, including Nivolumab, have been shown to be effective in metastatic RCC with a significant survival benefit (31-33). Radiation damage is believed to induce changes in the microenvironment and tumor antigen release that could augment the effects of immunomodulatory agents and work in a synergistic fashion. A Phase I study looking at the combination of SBRT and IL-2 found no dose limiting toxicities, and combination treatment appeared to have increased efficacy over the use of IL-2 alone (34). A Phase II study has since been performed considering this same combination in patients with RCC, and an interim report showed a 40% response rate, which is approximately twofold greater than historical controls (35). These results are exciting and suggest that SBRT may prove to be a powerful immune sensitizer for immunomodulatory agents. One of the main limitations to these trials however is the choice of IL-2, which given alone is known to have high rates of ≥ Grade 3 toxicity. In recent years checkpoint blockade inhibition with anti-CTLA-4 and anti-PD-1 antibody have been shown to be efficacious with lower rates of toxicity. This combination is a promising area for future research, with Australian researchers presently conducting a phase II clinical trial combining anti-PD-1 with SBRT for oligometastatic RCC (NCT02855203).
Technical considerations in precision radiation delivery
SBRT comprises the delivery of a single or a few fractions of ablative radiation treatments, delivered with high conformality and rapid dose fall-off to an extracranial target. Treatment units used to deliver SBRT include gantry-operated linear accelerators, CyberKnife (Accuray Inc., Sunnyvale, CA, USA) robotic radiosurgery system, Helical TomoTherapy, carbon ion therapy (36) and cyclotrons or synchrotrons used to deliver proton therapy. Planning techniques utilized by gantry-operated linear accelerators include 3-dimensional conformal radiotherapy (3D CRT), intensity modulated radiation therapy (IMRT), volumetric modulated arc therapy (VMAT), and dynamic conformal arcs. In terms of resource implications and workflow, linear accelerators have the advantage of being able to treat both stereotactic and non-stereotactic radiotherapy cases, and globally would be the most widely available type of treatment unit available in most radiotherapy departments. CyberKnife® is a purpose built stereotactic unit and comprises a compact linear accelerator with 12 different sized circular collimators mounted upon a robotic arm, with six degrees of positional freedom. In addition to its ability to efficiently deliver numerous non-coplanar beams, its accuracy is enhanced through the use of an optic image guidance system, which allows for both tracking and real-time correction of any tumor or patient movement through adjustment of the robotic arm. This is performed using two orthogonal X-ray sources attached to the ceiling, which capture orthogonal images that are regularly compared using fiducial markers or bony matching to the original digitally reconstructed radiographs (DRR) and adjusted in real time. For kidney and adrenal tumors, synchrony is used for tracking of tumor motion caused by respiration. The TomoTherapy HiArt System® (TomoTherapy, Madison, Wisconsin, USA) delivers IMRT during continuous 360-degree rotations, and can obtain megavoltage (MV) CT images prior to treatment delivery. While all these systems have the capability to deliver SBRT, with outcomes appearing to be similar across treatment platforms, they each have their own advantages and disadvantages, and other factors including local expertise, and resource implications should also be taken into consideration.
Regardless of the type of unit used to deliver SBRT, an important consideration for treating the kidney and adrenal gland using SBRT is motion management. The kidney and adrenal gland move with respiration and this needs to be quantified and accounted for, which may often be challenging. One review assessing kidney motion, unexpectedly found that the kidney moved the least in free breathing patients (between 4.5 to 13.9 mm), with a greater range of movement seen with the use of a compression device or the prone position (between 4.6 and 18.1 mm) and the use of deep breathing and breath hold techniques (between 10.1 to 41 mm) (37). A pilot study of nine patients in which fiducial markers were inserted near the adrenal gland revealed on average the markers moved 3.4, 5.4 and 9.9 mm in the left-right, anterior-posterior and cranio-caudal directions respectively. There was no difference between patients treated in the supine versus the prone position (25). One study utilized a dual vacuum stabilization device, which reduced kidney motion in six out of nine participants, however increased motion in one participant (38). Given the wide ranges seen in motion between patients and the difficulties with limiting free-breathing motion, when using an internal target volume (ITV) concept, a thin cut 4DCT should also be obtained during simulation and utilized. The ITV should subsequently be expanded by approximately 3–10 mm to generate a PTV (5). Respiratory gating or tumor tracking using implanted fiducial markers, if available, may be used to allow for a reduction in ITV, and this is usually incorporated into delivery of SBRT using CyberKnife®.
The target volume is usually defined using contrast enhanced CT, with the addition of MRI and PET scans in certain cases. MRI may be particularly useful in imaging the upper and lower poles of the kidneys and in determining invasion of vascular structures. FDG-PET although not frequently used in patients with RCC, has a sensitivity and specificity for metastatic RCC of 91% and 88% respectively and may be a useful modality in addition to CT if available (39). There is also emerging evidence as to the use of PSMA PET in RCC, with a pilot study of 10 patients with metastatic RCC, reporting two patients underwent treatment modification after PSMA PET, including one patient in whom vascular invasion was identified which was not seen on CT (40).
Several dose fractionation regimens have been used in primary RCC and adrenal metastases. Typically, doses employed in primary RCC commonly vary between 30 to 45 Gy in three to five fractions or single fraction regimes of 25–26 Gy (41,42). The International Radiosurgery Oncology Consortium for Kidney (IROCK) developed a set of international guidelines with the aim of standardizing treatment delivery, supported by results of a survey sent to eight international institutions (5). The guidelines report doses used in these institutions varied between 25–80 Gy in 1–12 fractions, and identified dose/fractionation as a key area of inconsistency that requires further investigation. One phase I dose escalation study escalated the radiation dose from 24 Gy successfully to 48 Gy in four fractions, finding this regimen safe and feasible, and further escalation to 60 Gy in three fractions is ongoing (43). The selection of a fractionation regimen outside of a study depends upon multiple factors including adjacent critical organs at risk (OAR) such as the contralateral kidney and small bowel, and tumor size, with larger targets usually being treated with more fractionated SBRT regimens. There is limited evidence to guide us regarding the appropriate dose that should be used for adrenal metastases. There is a wide spectrum of doses used in the literature, which is largely retrospective in nature. In one of the larger series, doses used varied between 21 to 54 Gy in 3 fractions (44). There is some evidence to support a correlation between LC and BED (45). Extrapolating from literature in lung SBRT, a BED10 of >100 Gy may be more effective (46) although further data is required to validate this in other settings.
Different techniques may be used to plan SBRT, depending upon the radiotherapy unit used. Beam number and direction will also vary depending upon the technique used, tumor size and position, and may be a combination of both coplanar and non-coplanar beams. In general, SBRT is usually prescribed to the 75–90% isodose line, aiming for adequate PTV coverage, while still achieving a steep dose gradient, and optimizing conformity, and thereby protecting adjacent OARs. A study of 20 patients treated with 3D CRT on a conventional linear accelerator showed that the number of beams required increased with the size of tumor (8 for PTV <100 cm3 and 10 for PTV >100 cm3) (41). In addition, the dose gradient (quantified by the intermediate dose spillage at 50% of the prescription dose, R50%) was inversely proportional to the number of beams used. A small study by Sonier et al. compared VMAT versus IMRT in delivery of SBRT for patients with either primary RCC or adrenal metastases (47) and found that VMAT performed better than IMRT with respect to treatment time (4 versus 13 minutes respectively), target homogeneity, 95% conformity index (1.32 versus 1.12), and maximum point dose to adjacent OARs, while VMAT performed worse with respect to low dose wash and intermediate dose to distant OARs. A study by Scorsetti et al. compared different treatment plans for adrenal metastases with protons and photons for 10 patients receiving a dose of 45 Gy in 7.5 Gy per fraction (48). Techniques assessed included VMAT, dynamic conformal arcs, 3D conformal static fields, IMRT, and intensity modulated protons. The most conformal plans were achieved with IMRT and VMAT, while the lowest V10 Gy and integral dose was achieved by protons. Ongoing studies will hopefully assist in informing guidelines and benchmarks for minimum PTV coverage, and conformity indices, that will improve and standardize delivery of SBRT.
Organ at risk constraints are another area in which our knowledge is still evolving. A critical issue in delivery of SBRT for primary RCC is ascertaining kidney tolerances to partial volume and high dose irradiation. Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) guidelines report that there is no established consensus for kidney dose constraints (49). IROCK guidelines provide consensus recommendations for OAR constraints (5), including to the contralateral kidney. The guidelines suggest for 3 fraction regimens to aim for V10 <33% while for all other fractionations to aim for as low as reasonably achievable (ALARA). No consensus could be reached for patients with a single kidney, although a recommendation to avoid as much normal parenchyma as possible was made. Estimated glomerular filtration rate (eGFR) measurements are usually recommended as an appropriate end point to measure renal function. However, more recently imaging is being utilized as it provides geographical and volumetric data regarding renal perfusion and associated kidney damage (50). Other critical OARs include the small bowel, large bowel, liver, spinal cord, lungs, and stomach and dose constraints for these organs vary depending upon the fractionation used, however the data supporting this is limited.
Image guidance is another key component of successful SBRT. While CyberKnife® allows for continuous on treatment imaging, with any variation being accounted for by adjustment of the robotic arm, image verification for linear accelerator based treatment usually requires pre-treatment image verification with cone beam CT for soft tissue matching to ITV/PTV, with intra-fractional monitoring also commonly utilized by many centers (5).
Efficacy and safety data for SBRT in RCC
The most recently published prospective clinical trial of 37 patients with inoperable primary RCC demonstrated SBRT to have excellent LC with low rates of toxicity (51). The majority of patients had T1b disease (>4 cm). The study used a dose of either 26 Gy in a single fraction or 42 Gy in 3 fractions for patients with disease ≥5 cm, and reported 89% of patients were able to complete treatment. Two-year freedom from local progression, distant progression, and overall survival rates were reported as 100%, 89% and 92%, respectively. Minor treatment related toxicities were reported in a large majority of patients (78%) with early Grade 1 fatigue and late Grade 1 chest wall pain being most commonly seen. Severe toxicities however were rare, with only 1 patient experiencing Grade 3 late fatigue, and no Grade 4 or 5 toxicities seen. Another recent prospective study was a Phase I dose escalation study by Ponsky et al. (2015), in which doses from 24 Gy to 48 Gy in 4 fractions were delivered to 19 patients (43). Median follow up for the study was 13.7 months. Stable disease was seen in 12 patients, and a partial response in three patients. Of 11 patients that had post treatment biopsies, two achieved negative results. Estimated 3-year OS post SBRT was 72%. Acute toxicities seen included one patient with Grade 2 fatigue and one with Grade 4 duodenal ulcer 44 days post treatment, which was thought to be potentially related to SBRT. In the latter case, the maximum point dose to the bowel was 54 Gy in 4 fractions. Treatment related late toxicities included one patient with Grade 2 urinary incontinence, two with Grade 3 renal toxicity (with eGRF of 15 and 16 mL/min/1.73 m2) and one patient with Grade 4 duodenal ulcer (same as patient with acute ulcer).
The findings of these studies are supported by a systematic review published in 2012 of 10 studies (including 3 prospective studies) comprising of 126 patients (16). The overall weighted LC was 93% and the weighted rate of ≥ Grade 3 toxicity was 4%. Follow up for most of the trials was 2–3 years. Since 2012, newer prospective studies also continue to report short to medium term high LC rates and low rates of toxicity (41-43,52). Overall the main acute toxicity reported in these studies are acute fatigue, nausea, radiation dermatitis and enteritis. Severe toxicities rates are much lower, however importantly include renal and skin toxicity and duodenal ulcer (36,43,53). A summary of the current evidence is found in Table 1.

Full table
The impact of SBRT on renal function is an important consideration. Encouragingly, the rates of dialysis post SBRT are low (50,54,55). There is some data to suggest that a dose response relationship exists, with minimal renal function deficit (measured by calculated chromium-51 EDTA GFR and DMSA SPECT split functional imaging), seen in patients receiving 10 Gy or less in a single fraction, and plateauing above 100 Gy (BED3) (50). In this study, the R50% conformity index correlated to GFR loss and this value could be reviewed and minimized in order to curtail any adverse effect upon kidney function. Renal atrophy, as defined by change in renal volume, is another measure of renal dysfunction that has been studied in relation to the effects of radiotherapy. A small study of 14 patients treated with SBRT total doses of 50 to 70 Gy, showed renal atrophy to significantly correlate with V20–V30 Gy in 10 fractions (56). The median change in irradiated kidney volume in this study was 160.4 to 137.1cc with an associated change in median creatinine levels from 1.1 mg/dL to a peak of 1.6 mg/dL. Additionally, the rate of renal atrophy was significantly lower in patients who underwent fiducial marker insertion. Overall there was no Grade 2 renal toxicity or hemodialysis reported.
Additionally, there is emerging data from small series that even in patients with a pre-existing renal insult, SBRT may be a safe strategy. One study, reporting on nine patients with a mean baseline eGFR of 52 mL/min who were deemed to be at high risk of requiring dialysis post-operatively, were treated instead with SBRT (50). While there was a significant reduction seen in eGFR following SBRT to 43ml/min, no patients required dialysis. Another small study of 3 patients with even poorer renal function, with eGFRs of between 17.51 and 34.79 mL/min, used CyberKnife®-based SBRT to deliver 40 Gy in 5 fraction for primary inoperable RCC (57). In this study, 1 patient with eGFR of 17.51 mL/min experienced a reduction in eGFR at 26 months to 12.28 mL/min, constituting renal failure, although lived dialysis free prior to this. She received an ipsilateral kidney V15 Gy of 28%. The other two patients experienced a small reduction of eGFR to just below 30 mL/min, however did not require dialysis. No patients experienced local failure, suggesting even in patients with severe CRF, SBRT may present an option in those ineligible for extirpative surgery. Patients with single kidneys may also safely receive SBRT, with a study on seven such patients reporting a moderate elevation in creatinine levels in only two patients to 160 µmol/L with no patients requiring dialysis (54). These small series provide encouraging results although further data is required regarding patients with pre-existing renal dysfunction and establishing safe OAR constraints to better predict renal outcomes following treatment.
Efficacy and safety data for SBRT in adrenal metastases
Similar to the setting of primary RCC, the majority of data on adrenal SBRT is retrospective with some prospective studies emerging. A systematic review from 2014 reported on the use of surgical treatment, stereotactic radiotherapy and percutaneous catheter ablation (PCA) as treatment options for patients with adrenal metastases (58). Overall in this review, 178 patients received SBRT, with 68% of patients diagnosed with primary lung cancer, and 48% of the cohort reported to have isolated disease. The 1-year LC ranged between 44% and 100%, and the weighted 2 year LC was 63% (range, 27% to 100%). Given the range of doses and small study numbers, it is difficult to comment on the existence of a dose response relationship. The largest series within the systematic review was of 48 patients, with a reported 2-year LC of 90%. Most patients in this study received 36 Gy in 3 fractions (BED10 79) prescribed to the covering 70% isodose, resulting in a maximum PTV dose of approximately BED10 137 Gy (44). Other studies reported 100% LC rates, using median prescribed BED10 of 86 Gy (59) and 77 Gy (25). More recent studies have shown similar LC to that reported in the systematic review (45,60,61). A summary of the current evidence is found in Table 2. Within the systematic review, the data for LC for PCA was too limited to compare across modalities, however the pooled 2 year LC and OS for adrenalectomy was higher than that seen in SBRT at 84% versus 63% and 46% versus 19%, respectively. In the context of this non-randomized comparison, a number of possible factors could explain the differences seen between surgery and radiotherapy, including patient selection, differences in baseline patient characteristics, metastatic burden (75% of surgical versus 48% of SBRT patients with isolated disease), and primary histology (68% surgical versus 33% RT patients with primary lung cancer), and the range of SBRT doses utilized (with BED10 as low as 28 Gy in some patients). Where reported, toxicities were generally mild, with no Grade 3–4 toxicities. Rates of acute and late Grade 2 GI toxicity varied between 6–22% and 5–18% respectively. One patient had late Grade 2 fatigue and one patient had late Grade 2 adrenal insufficiency. While these results are generally reassuring, a separate case report of a fatal gastric ulcer with SBRT and concurrent vinorelbine (62) suggests that caution must be applied in treating intra-abdominal disease with SBRT given the rare potential for severe GI adverse effect that may be seen.

Full table
Issues and controversies in post-treatment follow-up
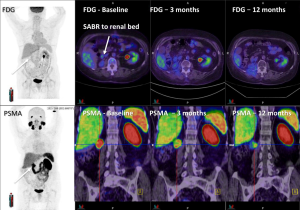
The aims of follow up include monitoring for adverse effects of treatment and cancer control. Following SBRT however, there are a number of considerations include timing, modality of follow up and definitions of LC that can present challenging issues for the clinician. LC is often measured using CT, and occasionally using MRI or PET, and variably using the response evaluation criteria in solid tumors (RECIST) system. The RECIST system however is a generalized system that is applied to all solid tumor measurements and does not take into account the specifics of the area being treated nor the modality of treatment. The American Urological Association (AUA) have developed a definition specific to the kidney in the context of post-surgical changes. While this is more specific than RECIST, it may be problematic when applied in the post SBRT setting (63). In particular the absence of enhancement, which may be applicable to surgery, and even RFA and cryotherapy (64) is not particularly useful following SBRT, as contrast enhancement changes often slowly evolve after SBRT (65). Size, while often used clinically, can be a crude measurement, and once again, not always relevant post radiotherapy, following which many tumors may not significantly reduce in size, or shrink over a period of many months to years, and occasionally may develop pseudoprogressive changes, with initial enlargement commonly in the immediate 3–6 months post-treatment (36). Whilst the biological mechanism of this initial change is not understood, it may be due to initial post-treatment inflammation secondary to very large dose per fraction radiation. Given these uncertainties, practical methods of addressing these issues in clinic and within trials, include delaying initial follow up imaging to approximately 6 months post treatment, and use of absence of progression rather than tumor regression as an endpoint to measure treatment success. Other strategies being explored include novel imaging modalities, such as diffusion weighted MRI, dynamic contrast enhanced MRI (66-68) and PET scans (see Figure 1) (65). Biopsy post treatment is another consideration, however interpretation and timing of biopsies also pose difficulties, as shown in one study in which 64% of RCC biopsies were positive 6 months after SBRT, yet failed to progress on subsequent imaging (43). Additionally, a case report of an autopsy performed 2.5 years following SBRT to 60 Gy in 10 fractions to a primary RCC, showed partial imaging response, with histopathological assessment showing almost complete tumor necrosis, although with a few viable tumor cells still present (69). The issue of biopsy timing in addition to imaging timing may be an important consideration and is being explored in a current trial (Clinical trials database: NCT02141919). Lastly serum biomarkers are increasingly being investigated and provide another avenue for follow up and may prove useful in the future (51).
RCC and inherent ‘radioresistance’—have we seen this all before?
Radiotherapy has typically been sidelined in the treatment of primary RCC, partly due to disappointing pre-clinical and randomized clinical study results that utilized 2 Gy per fraction and now outdated radiotherapy techniques (70-73). These studies suggested that RCC was inherently radioresistant and fostered nihilism amongst clinicians, which ignored the limitations of these studies particularly in the context of modern clinical practice. Technical advances and the implementation of SBRT, as well as a greater understanding of the radiobiology of RCC, have allowed radiotherapy to re-emerge as a viable and promising treatment option. Ablative dose per fraction radiotherapy activates a different apoptosis pathway compared to conventional radiotherapy, resulting in translocation of ASMase and formation of pro-apoptotic ceramide, which within one hour of radiotherapy delivery produces endothelial cell death, critical in the realization of tumor kill for vascular malignancies such as RCC (74-77). Conventional radiotherapy causes oxygen-dependent DNA damage and P53-mediated programmed cell death, which allows amassing of pro-angiogenic factors, and ongoing viability of the vascular endothelium. The effectiveness of high doses of radiotherapy, are further supported by pre-clinical studies, including a study on 12 nude mice with RCC (A498 cell line) which delivered 48 Gy in three weekly fractions and showed 30% tumor regression and no active mitoses in the irradiated mice, while the control mice showed tumor progression and up to 14 mitoses per high powered field at seven weeks (78). Cell survival curve studies also provide evidence for the use of stereotactic radiotherapy, including a study of two human RCC cell lines (A498 and Caki-1) which show the α/β ratio of RCC is relatively low (2.6 and 6.9 respectively) and therefore likely more sensitive to high fractional doses (79).
Future directions
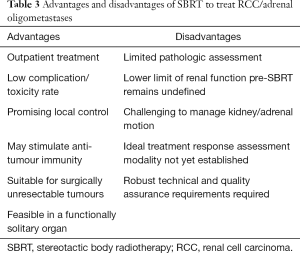
SBRT is a technique that is being rapidly incorporated worldwide into modern day radiotherapy practices. While the SBRT data is maturing in some tumor streams such as lung, evidence for SBRT in RCC and adrenal metastases is rapidly emerging although remains in relatively early stages. Although there are a number of advantages, there are some limitations that also need to be considered (see Table 3). There is an urgent need for long-term data from prospective trials for both patient-centric and objective measures of efficacy and safety. Our knowledge regarding patient selection is improving, however requires ongoing refinement. We also need to concentrate efforts on the development of sophisticated parameters, including biomarkers, and imaging technology for measuring outcomes, that better predict and quantify treatment response as well as organ function. The immunomodulatory effect requires further exploration, not only in the context of the abscopal effect, but in the potential of radiotherapy to augment and sensitize the effects of currently available immune modulating agents. Looking forward, ongoing and future studies will hopefully build upon the current body of literature to address any current gaps and allow clinicians and patients to continue to utilize and benefit from the use of SBRT.
Full table
Acknowledgements
Ian Vela is supported by a Movember Clinician Scientist Award.
Footnote
Conflicts of Interest: Alexander V. Louie has received honoraria from Varian Medical Systems Inc. The other authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Oshiro Y, Takeda Y, Hirano S, et al. Role of radiotherapy for local control of asymptomatic adrenal metastasis from lung cancer. Am J Clin Oncol 2011;34:249-53. [Crossref] [PubMed]
- Kumar R, Xiu Y, Yu JQ, et al. 18F-FDG PET in evaluation of adrenal lesions in patients with lung cancer. J Nucl Med 2004;45:2058-62. [PubMed]
- Mitchell IC, Nwariaku FE. Adrenal masses in the cancer patient: surveillance or excision. Oncologist 2007;12:168-74. [Crossref] [PubMed]
- Siva S, Ellis RJ, Ponsky L, et al. Consensus statement from the International Radiosurgery Oncology Consortium for Kidney for primary renal cell carcinoma. Future Oncol 2016;12:637-45. [Crossref] [PubMed]
- Correa RJ, Rodrigues GB, Chen H, et al. Stereotactic Ablative Radiotherapy (SABR) for Large Renal Tumors: A Retrospective Case Series Evaluating Clinical Outcomes, Toxicity, and Technical Considerations. Am J Clin Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Swaminath A, Chu W. Stereotactic body radiotherapy for the treatment of medically inoperable primary renal cell carcinoma: Current evidence and future directions. Can Urol Assoc J 2015;9:275-80. [Crossref] [PubMed]
- Siva S, Daniels CP, Ellis RJ, et al. Stereotactic ablative body radiotherapy for primary kidney cancer: what have we learned from prospective trials and what does the future hold? Future Oncol 2016;12:601-6. [Crossref] [PubMed]
- Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer 2008;113:2671-80. [Crossref] [PubMed]
- Lagerveld BW, Brenninkmeijer M, van der Zee JA, et al. Can RENAL and PADUA nephrometry indices predict complications of laparoscopic cryoablation for clinical stage T1 renal tumors? J Endourol 2014;28:464-71. [Crossref] [PubMed]
- Kim EH, Tanagho YS, Bhayani SB, et al. Percutaneous cryoablation of renal masses: Washington University experience of treating 129 tumours. BJU Int 2013;111:872-9. [Crossref] [PubMed]
- Motzer RJ, Bander NH, Nanus DM. Renal-Cell Carcinoma. N Engl J Med 1996;335:865-75. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 2004;171:1071-6. [Crossref] [PubMed]
- Daliani DD, Tannir NM, Papandreou CN, et al. Prospective assessment of systemic therapy followed by surgical removal of metastases in selected patients with renal cell carcinoma. BJU Int 2009;104:456-60. [Crossref] [PubMed]
- Siva S, Pham D, Gill S, et al. A systematic review of stereotactic radiotherapy ablation for primary renal cell carcinoma. BJU Int 2012;110:E737-43. [Crossref] [PubMed]
- Soffen EM, Solin LJ, Rubenstein JH, et al. Palliative radiotherapy for symptomatic adrenal metastases. Cancer 1990;65:1318-20. [Crossref] [PubMed]
- Zeng ZC, Tang ZY, Fan J, et al. Radiation therapy for adrenal gland metastases from hepatocellular carcinoma. Jpn J Clin Oncol 2005;35:61-7. [Crossref] [PubMed]
- Mittendorf EA, Lim SJ, Schacherer CW, et al. Melanoma adrenal metastasis: natural history and surgical management. Am J Surg 2008;195:363-8; discussion 368-9. [Crossref] [PubMed]
- Muth A, Persson F, Jansson S, et al. Prognostic factors for survival after surgery for adrenal metastasis. Eur J Surg Oncol 2010;36:699-704. [Crossref] [PubMed]
- Luketich JD, Burt ME. Does resection of adrenal metastases from non-small cell lung cancer improve survival? Ann Thorac Surg 1996;62:1614-6. [Crossref] [PubMed]
- Tanvetyanon T, Robinson LA, Schell MJ, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: a systematic review and pooled analysis. J Clin Oncol 2008;26:1142-7. [Crossref] [PubMed]
- Eldaya RW, Paulino AC, Blanco AI, et al. Preservation of adrenal function after successful stereotactic body radiation therapy of metastatic renal cell carcinoma involving the remaining contralateral adrenal gland. Pract Radiat Oncol 2012;2:270-3. [Crossref] [PubMed]
- Leenstra JL, Tanaka S, Kline RW, et al. Factors associated with endocrine deficits after stereotactic radiosurgery of pituitary adenomas. Neurosurgery 2010;67:27-32; discussion 32-3.
- Katoh N, Onimaru R, Sakuhara Y, et al. Real-time tumor-tracking radiotherapy for adrenal tumors. Radiother Oncol 2008;87:418-24. [Crossref] [PubMed]
- Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953;26:234-41. [Crossref] [PubMed]
- Chen AC, Butler EB, Lo SS, et al. Radiotherapy and the abscopal effect: insight from the past, present, and future. J Radiat Oncol 2015;4:321-30. [Crossref]
- Ishiyama H, Teh BS, Ren H, et al. Spontaneous regression of thoracic metastases while progression of brain metastases after stereotactic radiosurgery and stereotactic body radiotherapy for metastatic renal cell carcinoma: abscopal effect prevented by the blood-brain barrier? Clin Genitourin Cancer 2012;10:196-8. [Crossref] [PubMed]
- Reynders K, Illidge T, Siva S, et al. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015;41:503-10. [Crossref] [PubMed]
- Siva S, MacManus MP, Martin RF, et al. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett 2015;356:82-90. [Crossref] [PubMed]
- Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 2003;21:3127-32. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol 2015;33:1430-7. [Crossref] [PubMed]
- Seung SK, Curti BD, Crittenden M, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses. Sci Transl Med 2012;4:137ra74. [Crossref] [PubMed]
- Hannan R, Ishihara D, Louder K, et al., editors. Phase II trial of high-dose interleukin-2 (IL-2) and stereotactic radiation therapy (SABR) for metastatic clear cell renal cell carcinoma (ccRCC): Interim analysis. ASCO Annual Meeting Proceedings; 2016.
- Nomiya T, Tsuji H, Hirasawa N, et al. Carbon ion radiation therapy for primary renal cell carcinoma: initial clinical experience. Int J Radiat Oncol Biol Phys 2008;72:828-33. [Crossref] [PubMed]
- Pham D, Kron T, Foroudi F, et al. A review of kidney motion under free, deep and forced-shallow breathing conditions: implications for stereotactic ablative body radiotherapy treatment. Technol Cancer Res Treat 2014;13:315-23. [Crossref] [PubMed]
- Pham D, Kron T, Styles C, et al. The Use of Dual Vacuum Stabilization Device to Reduce Kidney Motion for Stereotactic Radiotherapy Planning. Technol Cancer Res Treat 2015;14:149-57. [Crossref] [PubMed]
- de Llano SM, Delgado-Bolton R, Jiménez-Vicioso A, et al. Meta-analysis of the diagnostic performance of 18F-FDG PET in renal cell carcinoma. Rev Esp Med Nucl 2007;26:19-29. [PubMed]
- Rhee H, Blazak J, Tham CM, et al. Pilot study: use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res 2016;6:76. [Crossref] [PubMed]
- Pham D, Thompson A, Kron T, et al. Stereotactic ablative body radiation therapy for primary kidney cancer: a 3-dimensional conformal technique associated with low rates of early toxicity. Int J Radiat Oncol Biol Phys 2014;90:1061-8. [Crossref] [PubMed]
- Staehler M, Bader M, Schlenker B, et al. Single fraction radiosurgery for the treatment of renal tumors. J Urol 2015;193:771-5. [Crossref] [PubMed]
- Ponsky L, Lo SS, Zhang Y, et al. Phase I dose-escalation study of stereotactic body radiotherapy (SBRT) for poor surgical candidates with localized renal cell carcinoma. Radiother Oncol 2015;117:183-7. [Crossref] [PubMed]
- Casamassima F, Livi L, Masciullo S, et al. Stereotactic radiotherapy for adrenal gland metastases: university of Florence experience. Int J Radiat Oncol Biol Phys 2012;82:919-23. [Crossref] [PubMed]
- Desai A, Rai H, Haas J, et al. A Retrospective Review of CyberKnife Stereotactic Body Radiotherapy for Adrenal Tumors (Primary and Metastatic): Winthrop University Hospital Experience. Front Oncol 2015;5:185. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]
- Sonier M, Chu W, Lalani N, et al. Implementation of a volumetric modulated arc therapy treatment planning solution for kidney and adrenal stereotactic body radiation therapy. Med Dosim 2016;41:323-8. [Crossref] [PubMed]
- Scorsetti M, Mancosu P, Navarria P, et al. Stereotactic body radiation therapy (SBRT) for adrenal metastases: a feasibility study of advanced techniques with modulated photons and protons. Strahlenther Onkol 2011;187:238-44. [Crossref] [PubMed]
- Dawson LA, Kavanagh BD, Paulino AC, et al. Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys 2010;76:S108-15. [Crossref] [PubMed]
- Siva S, Jackson P, Kron T, et al. Impact of stereotactic radiotherapy on kidney function in primary renal cell carcinoma: Establishing a dose-response relationship. Radiother Oncol 2016;118:540-6. [Crossref] [PubMed]
- Siva S, Pham D, Kron T, et al. Stereotactic ablative body radiotherapy for inoperable primary kidney cancer: a prospective clinical trial. BJU Int 2017. [Crossref] [PubMed]
- Chang JH, Cheung P, Erler D, et al. Stereotactic Ablative Body Radiotherapy for Primary Renal Cell Carcinoma in Non-surgical Candidates: Initial Clinical Experience. Clin Oncol (R Coll Radiol) 2016;28:e109-14. [Crossref] [PubMed]
- McBride S, Wagner A, Kaplan I. A phase 1 dose-escalation study of robotic radiosurgery in inoperable primary renal cell carcinoma. Int J Radiat Oncol Biol Phys 2013;87:S84. [Crossref]
- Svedman C, Karlsson K, Rutkowska E, et al. Stereotactic body radiotherapy of primary and metastatic renal lesions for patients with only one functioning kidney. Acta Oncol 2008;47:1578-83. [Crossref] [PubMed]
- Jackson P, Foroudi F, Pham D, et al. Short communication: timeline of radiation-induced kidney function loss after stereotactic ablative body radiotherapy of renal cell carcinoma as evaluated by serial 99m Tc-DMSA SPECT/CT. Radiat Oncol 2014;9:1. [Crossref] [PubMed]
- Yamamoto T, Kadoya N, Takeda K, et al. Renal atrophy after stereotactic body radiotherapy for renal cell carcinoma. Radiat Oncol 2016;11:72. [Crossref] [PubMed]
- Lo CH, Huang WY, Chao HL, et al. Novel application of stereotactic ablative radiotherapy using CyberKnife® for early-stage renal cell carcinoma in patients with pre-existing chronic kidney disease: Initial clinical experiences. Oncol Lett 2014;8:355-60. [PubMed]
- Gunjur A, Duong C, Ball D, et al. Surgical and ablative therapies for the management of adrenal ‘oligometastases’–a systematic review. Cancer Treat Rev 2014;40:838-46. [Crossref] [PubMed]
- Ahmed KA, Barney BM, Macdonald OK, et al. Stereotactic body radiotherapy in the treatment of adrenal metastases. Am J Clin Oncol 2013;36:509-13. [Crossref] [PubMed]
- Franzese C, Franceschini D, Cozzi L, et al. Minimally Invasive Stereotactical Radio-ablation of Adrenal Metastases as an Alternative to Surgery. Cancer Res Treat 2017;49:20-8. [Crossref] [PubMed]
- Jung J, Yoon SM, Park HC, et al. Radiotherapy for Adrenal Metastasis from Hepatocellular Carcinoma: A Multi-Institutional Retrospective Study (KROG 13-05). PloS One 2016;11:e0152642. [Crossref] [PubMed]
- Onishi H, Ozaki M, Kuriyama K, et al. Serious gastric ulcer event after stereotactic body radiotherapy (SBRT) delivered with concomitant vinorelbine in a patient with left adrenal metastasis of lung cancer. Acta Oncol 2012;51:624-8. [Crossref] [PubMed]
- Donat SM, Diaz M, Bishoff JT, et al. Follow-up for Clinically Localized Renal Neoplasms: AUA Guideline. J Urol 2013;190:407-16. [Crossref] [PubMed]
- Iannuccilli JD, Grand DJ, Dupuy DE, et al. Percutaneous Ablation for Small Renal Masses—Imaging Follow-Up. Semin Intervent Radiol 2014;31:50-63. [Crossref] [PubMed]
- Siva S, Callahan J, Pryor D, et al. Utility of 68 Ga prostate specific membrane antigen - positron emission tomography in diagnosis and response assessment of recurrent renal cell carcinoma. J Med Imaging Radiat Oncol 2017;61:372-8. [Crossref] [PubMed]
- Parameswaran B, Lau E, Bergen N, et al. Dynamic contrast enhanced MR evaluation of inoperable renal tumours treated with stereotactic radiation: preliminary results: daunting but worthwhile? J Med Imaging Radiat Oncol 2013;57:156. [PubMed]
- Kang SK, Zhang A, Pandharipande PV, et al. DWI for Renal Mass Characterization: Systematic Review and Meta-Analysis of Diagnostic Test Performance. AJR Am J Roentgenol 2015;205:317-24. [Crossref] [PubMed]
- Jeon TY, Kim CK, Kim JH, et al. Assessment of early therapeutic response to sorafenib in renal cell carcinoma xenografts by dynamic contrast-enhanced and diffusion-weighted MR imaging. Br J Radiol 2015;88:20150163. [Crossref] [PubMed]
- Onishi H, Kawasaki T, Zakoji H, et al. Renal cell carcinoma treated with stereotactic radiotherapy with histological change confirmed on autopsy: a case report. BMC Res Notes 2014;7:270. [Crossref] [PubMed]
- Deschavanne PJ, Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys 1996;34:251-66. [Crossref] [PubMed]
- van der Werf-Messing B. Proceedings: Carcinoma of the kidney. Cancer 1973;32:1056-61. [Crossref] [PubMed]
- Juusela H, Malmio K, Alfthan O, et al. Preoperative irradiation in the treatment of renal adenocarcinoma. Scand J Urol Nephrol 1977;11:277-81. [Crossref] [PubMed]
- Tunio MA, Hashmi A, Rafi M. Need for a new trial to evaluate postoperative radiotherapy in renal cell carcinoma: a meta-analysis of randomized controlled trials. Ann Oncol 2010;21:1839-45. [Crossref] [PubMed]
- Sathishkumar S, Boyanovsky B, Karakashian AA, et al. Elevated sphingomyelinase activity and ceramide concentration in serum of patients undergoing high dose spatially fractionated radiation treatment: implications for endothelial apoptosis. Cancer Biol Ther 2005;4:979-86. [Crossref] [PubMed]
- Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003;300:1155-9. [Crossref] [PubMed]
- Li J, Yu W, Tiwary R, et al. alpha-TEA-induced death receptor dependent apoptosis involves activation of acid sphingomyelinase and elevated ceramide-enriched cell surface membranes. Cancer Cell Int 2010;10:40. [Crossref] [PubMed]
- De Meerleer G, Khoo V, Escudier B, et al. Radiotherapy for renal-cell carcinoma. Lancet Oncol 2014;15:e170-7. [Crossref] [PubMed]
- Walsh L, Stanfield JL, Cho LC, et al. Efficacy of ablative high-dose-per-fraction radiation for implanted human renal cell cancer in a nude mouse model. Eur Urol 2006;50:795-800; discussion 800. [Crossref] [PubMed]
- Ning S, Trisler K, Wessels BW, et al. Radiobiologic studies of radioimmunotherapy and external beam radiotherapy in vitro and in vivo in human renal cell carcinoma xenografts. Cancer 1997;80:2519-28. [Crossref] [PubMed]


