Updated response assessment criteria for high-grade glioma: beyond the MacDonald criteria
Introduction
High-grade glioma remains a highly challenging disease for neuro-oncologists and neurosurgeons alike, with a dismal prognosis. Despite decades of research and development, the median survival of patients with newly diagnosed glioblastoma multiforme (GBM) remains poor, with a range from 12 to 14 months (1). Maximal safe surgical resection followed by concurrent chemoradiotherapy [temozolomide (TMZ) with radiation therapy (RT)] and adjunct chemotherapy (TMZ) remains the current standard of care in these patients, with little change since the advent of the Stupp protocol in 2005 (1). Prognosis is even poorer in patients with recurrent GBM, with a 6-month PFS of approximately only 15% and a median overall survival (OS) ranging between 24–40 weeks (2,3). Consequently, there is a clear and urgent need for further development and investigation of promising new therapies, with a focus on identifying those therapies which are most likely to have a significant impact on GBM outcome. Tumor response to various therapeutic interventions is typically assessed using different imaging modalities, but intra-axial tumors such as GBM are most often evaluated using contrast enhanced magnetic (4). While imaging response is critical, other modalities have been incorporated in the assessment of treatment response. In their seminal paper published in 1977, Levin et al. (5) reported an equal predictive value of neurological examination, radionuclide scintiscan, CT scan and electroencephalogram (EEG) when used individually in determining tumor response to chemotherapy in 100 patients with malignant brain tumors. However, combining two of three modalities (i.e., neurological examination, CT scan, radionuclide scintiscan, etc.) conferred an improved predictive value, resulting in predicted response to therapy in 82% of patients (5). Taken together, these results would suggest that comprehensive response criteria should include multi-modal assessment to achieve a more accurate assessment of response.
Tumor response criteria
In 1981, the World Health Organization (WHO) published their first tumor response criteria, defining response to therapy as a change in the product of bidimensional tumor measurements while on treatment. This criterion was vague and was subsequently modified in several forms, aimed to address tumors in general irrespective of tumor type or location (6). In 1990, Macdonald et al. (7) reported a novel criteria to assess tumor response based on the combination of two dimensional tumor measurement on contrast enhanced CT or MRI, clinical status, and change in corticosteroid requirement following treatment. Based on these parameters, they classified response as complete, partial, stable and progressive disease (PD) (7). Complete response (CR) was defined as disappearance of all enhancing tumor off steroids, partial response (PR) as reduction of ≥50% on stable or reduced steroid dose, PD as ≥25% increase in size on stable or increased steroids, and stable disease as all other situations. Furthermore, the criteria incorporated clinical outcomes, requiring that patients be additionally neurologically stable or improved in order to qualify as PR or CR, and conversely that neurologic decline be categorized as PD. Importantly, the response by the Macdonald criteria required scans at least 1 month apart with reductions of greater than 50% without an increase in steroid use, in an attempt to identify sustained, significant reductions in tumor size independent of response to steroid (7). At the time, when some patients were followed by CT scans and even the MRI resolution was not what it is today, this stringent criterion was intended to eliminate the chance that the margin of error of tumor volume measurements would result in misclassification as a true response. Due to the objectivity of the Macdonald criteria in determining the response to therapy, these criteria were widely accepted in different clinical trials to make comparisons across different therapeutic interventions. These criteria have also been extensively used in recent clinical trials for Response Assessment in Neuro-Oncology (RANO) (8).
In 2000, the International Working Party published new criteria, known as Response Evaluation Criteria in Solid Tumors (RECIST), aiming to simplify and standardize the evaluation of solid tumor treatment response. The RECIST criteria differed from the original WHO tumor response criteria by calling for unidimensional instead of bidimensional measurements to assess tumor burden, defining a minimum size for measurable lesions, and creating a limit of ten lesions to follow in the case of metastatic disease, with only five in a single organ. Several prospective studies validated the replacement of bidimensional with unidimensional measurements, which further translated well to phase II studies of solid tumors. A RECIST Working Group was formed to regularly update these criteria based on new evidence, and revised guidelines have included modifications such as assessing tumor size using the sum of the diameters of each lesion in a given target tissue. Based on these criteria, PR is defined as ≥30% decrease in this sum; with PD defined as a 20% increase in sum requiring an absolute increase of 5 mm or appearance of a new lesion. The use of a unidimensional measurement and the summation of multiple lesions may have less relevance for intracranial malignancy in which the complex morphology of these tumors, and their tendency to infiltrate rather than forming new discrete lesions, limits the utility of the RECIST criteria. While these criteria have since been applied sporadically to high-grade glioma, it was ultimately designed with non-CNS solid-tumors in mind, and as a result has not been widely accepted by the neuro-oncology community (6,8).
While both the MacDonald and RECIST criteria have contributed to the standardization of evaluating tumor response, they both have notable limitations in the current treatment of high-grade glioma/GBM. Specifically, the MacDonald criteria is based entirely on enhancing tumor dimensions, which may be confounded in both directions by treatment-related enhancement changes, known as pseudoprogression (PsP), and alternatively by infiltrative disease beyond the areas of enhancement. The RECIST criteria, while useful for solid tumor malignancies, does not incorporate enhancement patterns or volume in its assessment, which may be critical in the case of GBM. Given these shortcomings along with advancement in neuroimaging, it was deemed necessary to redefine the endpoint assessment criteria in Neuro-Oncology. A multinational working group was formed to re-evaluate and re-define these criteria, termed the RANO criteria (9).
RANO criteria for glioma
Based on contrast enhanced MRI, the RANO committee laid down the following criteria for response assessment which are summarized in Table 1.

Full table
The RANO committee is actively working to establish guidelines for end-point assessment that can further be applied beyond high-grade glioma (15,16), to patients with brain metastases (17,18) and low-grade gliomas (19) (Table 2). These guidelines will be discussed in detail in following sections.
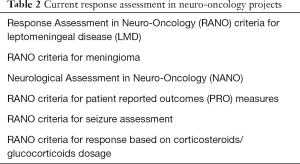
Full table
Imaging and clinical criteria used for RANO assessment
MRI T1W gadolinium (Gd) contrast enhancing imaging
Based on T1W Gd-contrast enhancing images (slices 5 mm apart with 0 mm skip), measurable disease is defined as enhancing lesions with well-defined margins and two perpendicular diameters of ≥10 mm. As detailed previously, cystic and necrotic regions of the tumor or surgical cavity are considered non-measurable, unless they demonstrate wall enhancement that exceeds 10 mm. Lesions which can be measured in only one dimension, with ill-defined margins, or maximum dimension <10 mm are also considered as non-measurable disease. Based on abovementioned criteria, patients with complete resection of enhancing tumor on post-operative scan can be considered only for studies looking at OS or PFS as end-points, due to the lack of measurable imaging response to follow (9). Despite this, patients having undergone a complete resection can still be followed for recurrence, and thereby “progression” (20,21).
An additional challenge in assessing response based on contrast enhancement frequently arises in patients who have undergone investigational surgical interventions such as convection-enhanced delivery (CED) of therapeutic agents (gene or immune), chemotherapy wafers, and radiosurgery, which can result in changes in enhancement independent of tumor response (22,23). In these patients in particular, it becomes critical to differentiate between true and pseudo tumor progression. PsP often appears as a contrast-enhancing lesion on MRI, and is seen in up to 20–30% of patients following concomitant chemoradiation therapy for GBM (particularly in MGMT methylated tumors) (10,24,25). These treatment-induced changes following chemoradiation appear to peak between 3 (26) and 6 months (27), which forms the basis of the RANO criteria stipulating a timeframe of 12 weeks. However, PsP can still occur outside of the stated window and remains a potential confounding variable affecting response assessment (28).
Although disease measurement using RANO criteria is similar to that described by Macdonald criteria (two-dimensional) and different from that described by RECIST criteria (one-dimensional) (7,9,29), various studies have shown strong correlation between these methods and tumor response (29-31). Two-dimensional measurement of tumor response has been widely adopted in clinical trials (20); yet there are intuitive advantages to 3D and volumetric tumor measurements in their ability to more effectively capture minor changes in the tumor dimensions following treatment, and to further quantify more subtle changes in non-measurable enhancing disease. The increased accuracy conferred by volumetric measurements becomes more apparent in the case of non-uniform tumors that take on complex shapes in a three-dimensional space, as in the case of intracranial tumors such as glioma, as well as tumors with mixed morphology including cysts, cavitation, and necrosis, and finally in multifocal or recurrent tumors (32). Another advantage of volumetric or 3D tumor measurement is that these measurements are independent of head position during image acquisition between serial follow up scans, which otherwise may lead to erroneous two-dimensional measurements (33). However, despite these benefits, volumetric assessment of tumor volumes is labor intensive and requires manual drawing of tumor volumes slice by slice on different software platforms, which may be challenging in routine clinical practice and perhaps more difficult to standardize (32,34). Nevertheless, with the inevitable ongoing advances in software platforms that streamline the measurement process, volumetric assessment of tumor response following treatment is likely to become the standard of care in near future.
MRI T2W/FLAIR imaging
An increasing use of anti-angiogenic therapeutic agents such as bevacizumab in patients with recurrent GBM (34,35) has led to several challenges in terms of response assessment on follow up imaging due to the impact of these agents on contrast enhancement, which unlike other therapies can lead to an artificial reduction in enhancement (Figure 1). These anti-VEGF agents reduces the vascular permeability of abnormally leaky vessels in patients with GBM leading to a significant decrease in contrast enhancement on follow up imaging, known as pseudo response (36,37). This pseudoresponse led to a significant objective response rate (ORR) of 29–42% using bevacizumab on MR imaging (38,39), without a significant impact on OS in patients with high-grade glioma (39-41). This dissociation between imaging response and clinical response suggests that in these patients, enhancement is not a reliable marker for tumor burden.
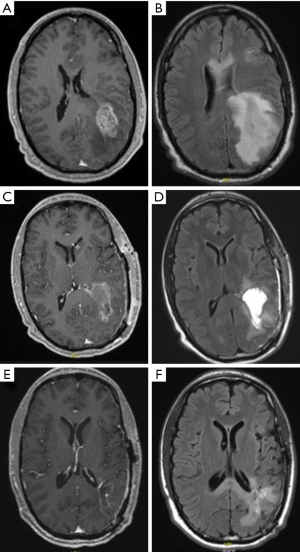
Patients on anti-angiogenic therapy typically demonstrate this pseudo response for a minimum of 4 weeks on follow up MRI (9). Conversely, anti-angiogenic agents reduce the incidence of pseudo progression, and consequently any evidence of increased enhancement in patients on these agents can be considered true tumor progression (42). Furthermore, patients on anti-angiogenic therapy for GBM have a very different pattern of tumor recurrence or progression, being more infiltrative and non-enhancing (5–10%) on MRI than otherwise (40,43-48), although there is no evidence to suggest that this has an impact on overall outcomes in patients on anti-angiogenic therapy for GBM (29,48-53). Given these complex changes resulting from anti-angiogenic therapy, to assess tumor response and recurrence/progression in patients who are on anti-angiogenic therapy for recurrent high-grade glioma, it is prudent to look beyond the contrast enhancement on MRI images using T2/FLAIR images (39).
Based on these unique challenges and the complex enhancement changes resulting from treatment, RANO committee has crucially integrated T2/FLAIR imaging as a part of tumor response assessment criteria. Based on T2/FLAIR imaging, tumor progression is defined as a significant increase in the dimensions of non-enhancing lesion, which cannot be attributed to radiation related changes, ischemic injury, infection, demyelination, seizures, post-operative and other treatment related changes (9). However, despite these guidelines, it is often a challenge to differentiate between true tumor progression and treatment effects based on changes seen on T2W/FLAIR MRI images. This challenge is more pronounced in the setting of patients with recurrent GBM who have been previously treated with multiple treatment modalities such as surgery, chemotherapy and RT including stereotactic radiosurgery.
To address the issues that arises with non-tumor causes of T2/FLAIR changes, RANO investigators attempted to define changes pointing towards true tumor progression. Features such as involvement of the cortical ribbon, non-enhancing lesion beyond the radiation field, presence of mass effect, signal intensity between gray matter and vasogenic edema, non-uniform signal intensity, and lack of typical finger-like projections suggestive of edema are advocated as findings more accurately pointing towards true tumor progression (47). The RANO investigators further aimed to define which changes are considered significant in an effort to elucidate the difference between tumor and non-tumor causes of T2/FLAIR changes. The RANO committee has also attempted to standardize methods of quantification on T2/FLAIR images to facilitate comparison on serial follow-up imaging (54-56). Techniques such as quantitative maps of differential T2 relaxation times between the normal brain, edematous brain and infiltrative tumor have been generated prior to and during therapy in order to subtract out the non-specific causes of signal change to identify areas of true tumor progression (57,58). Voxel-based, pre- and post-T2 subtraction analysis displayed in color-coded maps have been utilized to define the extent of true tumor progression as well as to quantify the effect of antiangiogenic treatment on edema demonstrated on T2/FLAIR images (54,56). However, further studies are needed to demonstrate consistent correlation between changes on the T2 relaxation maps and its impact on survival in order to avoid over or under-estimation of treatment effect based on these newer methods.
Positron emission tomography (PET) scan
Given the limitations of MRI imaging in the assessment of tumor response following anti-angiogenic treatment detailed above, 18F-fluorodeoxy-glucose (18F-FDG) PET, 18F-fluorothymidine (18F-FLT) PET and 18F-fluoroethyl-L-tyrosine (18F-FET) PET scans have been explored in various studies as an alternate method to evaluate tumor response to various antiangiogenic therapies. 18F-FLT-PET scans have been shown to identify tumor response earlier than standard anatomical contrast enhanced MRI and are also predictive of both OS and PFS (59). Similarly, 18F FDOPA PET scans have significant correlation between changes in uptake during anti-angiogenic therapy and OS (60).
The RANO working group in association with European Neuro-Oncology society (EANO) recently published guidelines regarding the clinical use of PET in patients with glioma (61). Amino acid based PET scans (FET and 18F DOPA) allow for increased tumor differentiation, target definition and follow up response when compared to glucose based PET scan (18F-FDG). Although amino acid PET scans have been shown to have better sensitivity, specificity and accuracy in differentiating glioma and inflammatory tissues compared to 18F-FDG-PET scan, both modalities have notable overlap in uptake values in low and high-grade glioma. However, overall, there is increased uptake in higher-grade glioma (WHO III/IV) compared to lower grade lesions (WHO II). Dynamic analysis of 18F-FET PET scans can augment discerning between high and low grade gliomas. Similar to other PET modalities, 18F DOPA PET scan has also been found to be useful in assessing response in patients on antiangiogenic therapy. Finally, amino acid PET improves the diagnostic accuracy of pseudo progression, progression and radiation necrosis (61).
Despite the evident usefulness of these modalities as an adjunct in assessing the tumor response, the widespread applicability of these PET scans are often limited by availability, lack of trained personnel and cost associated with these procedures.
Standardized brain tumor imaging protocol (BTIP)
A recent meeting hosted by the National Cancer Institute which included representatives from the FDA, multiple brain tumor interest groups, radiologists, and a select group of Neuro-Oncology experts yielded recommendations for a standardized BTIP intended to be used in multicenter therapeutic studies to evaluate the efficacy of treatments in patients with malignant brain tumors (62). The standardization in imaging protocol more readily allows for pooling and rapid comparisons of data from the multiple centers participating in brain tumor clinical trials. Recommendations included a minimum of pre contrast 3DT1w (IR-GRE), axial 2D FLAIR (TSE), axial 2D DWI and post contrast axial 2DT2w (TSE), 3DT1w (IR-GRE) for 1.5 T and 3 T MRI scans. These recommendations advocated additional 2DT1w, GRET2 scans, etc. on a tailored basis (62). This protocol has since been rapidly incorporated in the majority of new clinical trials involving malignant glioma. Currently, there are no guidelines for acquiring diffusion tensor imaging (DTI) in brain tumor trials. In general, 30–32 diffusion directions with one b-value (1,000 s/mm2) and minimizing TE (60–100 ms) yield reproducible fractional anisotropy (FA) and apparent diffusion coefficient (ADC) images (63,64).
Role of corticosteroids/glucocorticoids
Glucocorticoids have been used in routine clinical practice to reduce tumor-associated edema and improve neurological functions in patients, particularly in those with newly diagnosed or recurrent high-grade gliomas (1,38). Dexamethasone has been shown to decrease tumor-associated edema in a dual fashion by reducing the expression of vascular permeability factor (VPF) by the tumor cells, while also minimizing the ability of the vasculature to respond to these vasoactive factors (65). In vitro studies also have demonstrated that glucocorticoids inhibit the proliferation, migration and invasion of glioma (66). However, despite these benefits, given the well-described morbidity and intolerance to long-term steroid use, a reduction in the requirement of steroid dose during treatment is often considered as a favorable response to the given treatment modality (9).
In the BRAIN study involving patients with recurrent GBM treated with bevacizumab, the authors attempted to evaluate the use of corticosteroids during treatment. Response required ≥50% reduction in corticosteroid dose for ≥50% of the time while on study medication or complete discontinuation of corticosteroids for ≥25% of time (67), resulting in sustained reduction in 30.2% of patients with 16.3% of patients sustained off steroids altogether while on bevacizumab. Another Phase III randomized, partially blinded clinical trial (REGAL) compared treatment with cediranib monotherapy, lomustine monotherapy, or combined treatment and reported the results as a function of corticosteroid use. In this study, both cediranib monotherapy and combination therapy (cediranib plus lomustine arm) resulted in a significant decrease in steroid use (26% and 23% reduction from baseline respectively) compared to 5% increase seen with lomustine monotherapy alone (68). The results of such studies emphasize the importance of incorporating the use of steroids as surrogate for clinical response. The RANO working committee is in the process of defining the criteria for steroid responders (complete and PR) in order to formulate formal RANO recommendations.
Assessment of neurological status
The importance of a thorough clinical neurological examination in evaluating the functional status of the patient and their overall health cannot be overemphasized (69). Acknowledging this, the RANO working committee included clinical status as a component of response assessment to the given treatment modality. Various assessment measures such as Karnofsky Performance Scale (KPS), Medical Research Council scale for Muscle Strength Council (MRC), common terminology criteria for adverse events (CTCAE), patient reported outcomes (PROs) [European Organization for Research and Treatment of Cancer Brain tumor module (EORTC-BN), MD Anderson Symptom Inventory (MDASI)] and neurocognitive test battery (Hopkins Verbal Learning Test-Revised: HVLT-R), Trail making test A and B and multilingual aphasia examination and oral word association test) have been utilized (18).
Unlike patient reported subjective assessment methods, patient performance on these neurocognitive scales is a reliable measure to assess and compare the overall neurological functioning of patients across different clinical studies (70,71). There are, however, several challenges in terms of applicability of these objective neurocognitive measures, such as variability in clinical presentations, frequency of these tests and variability in different studies, rapid disease progression in patients with recurrent GBM, patient compliance, strict adherence to protocol, floor and ceiling effects among different tests and comparability among these tests (18).
Taking into account these logistical issues, and in an attempt to create a standardized assessment protocol, the RANO committee has proposed that clinical trials should include a pre specified assessment schedule throughout the study, baseline cognitive assessments, measures to ensure patient and investigators compliance and adherence to the protocol, as well as protocols to stratify the patients based on their performance on these neurocognitive scales. It would be practical to perform these assessments in conjunction with follow-up imaging to minimize the visit burden to the patient and maximize compliance.
Neurological assessment in neuro-oncology (NANO)
The NANO working group created an objective scale to better assess clinical response and disease progression in an effort to generate consistency between future studies. The NANO scale is based on clinical assessment of domains such as level of consciousness, behavior, language, facial strength, visual fields, strength, sensation, ataxia (upper extremity) and gait. These domains are evaluated on a scale ranging from zero to three, with zero representing normal and three representing grossly abnormal. This scale is intended to be integrated into the standardized RANO criteria and to complement the evaluation of MRI progression with clinical outcome measures including quality of life (QOL), neurocognitive functions, system burden inventories, in order to create a more comprehensive picture of treatment outcome. In this scale, response requires significant improvement defined as ≥2 level change in at least one domain, without concomitant worsening in other domains. Neurological progression is defined as significant worsening with ≥2 level change in any one domain, or change to the highest score in that same domain. Stable disease encompasses those that do not meet criteria for either response or progression, while patients in whom there are limitations on the ability to perform an accurate neurologic assessment are classified as non-evaluable.
An international prospective study used the NANO scale domains during a scheduled office visit as a secondary outcome in an attempt to determine the variability between observers and to assess the practicality of performing these assessments. This study has reported the inter-observer agreement ranging between 90.7% and 99.5% (moderate to substantial strength of agreement) across various neurological domains in 220 patients (unpublished data, SNO meeting RANO update 2016). The group concluded based on these findings that the NANO scale can be easily incorporated into clinical practice and can reliably be performed not only by neurologists, but by other physicians as well. Currently, the NANO scale is included as an exploratory end-point in various phase I and II clinical trials in patients with GBM, brain metastasis and PCNSL.
RANO criteria for seizure assessment
Seizure outcome definitions based on the International League against Epilepsy (ILAE) (72) classification range from seizure freedom with no auras (Score 1) to greater than 100% increase from baseline seizure with or without auras (Score 6) (73). Given the propensity of certain tumors to cause seizures, particularly when enlarging or recurring, seizure control has been proposed as an outcome measure in determining treatment response in clinical trials of patients with low-grade glioma (74). This assessment further incorporates a variety of health related QOL and symptom burden scales (Fact-Br, BN20 and MDASI-BT) that emphasize symptoms and neurologic function (74). Seizure frequency is evaluated prior to the initiation of treatment (12 months to the most recent 3 months) and following treatment and stratified in 10% decrements (classes 4 and 5) or increments (classes 5 and 6) compared to baseline. Rare seizures are given a class 3 score. A composite score (2 to 19) is then calculated incorporating the seizure classification score (1 to 3), frequency score, ILAE outcome score (1 to 6) and MD Anderson symptom inventory score (MDASI, 0 to 10) (74). The composite score is then used to define CR as patients who are completely seizure free, while PR requires at least one level improvement on the outcome scale compared to baseline. The authors have proposed a pilot trial to assess the validity of this seizure assessment as a tool for measuring outcomes in conjunction with clinical assessment in patients with brain tumors.
RANO criteria for PRO measures
The objective of this international multidisciplinary working committee is to create standard PRO criteria in patients with a variety of intracranial tumors (glioma, brain metastasis meningioma and CNS lymphoma). These PROs are based on several subjective measures such as clinical symptoms, functions and health related QOL measures from patient’s perspective. They are further tailored to the type of study being performed. This committee works with the other RANO committees as well as external committees in order to generate study design recommendations that take into account PROs. In this capacity this group aims to systematically review the use of PRO measures in prior brain tumor studies and to review the validity and reliability of using these measures in order to create standardized guidelines for using PRO measures in clinical trials for brain tumors (75).
Clinical application of RANO criteria for gliomas
With regard to end-points for response assessment to evaluate therapeutic efficacy, parameters such as ORR, OS and progression free survival (PFS) have been used traditionally in various studies.
ORR is an early end-point based on the radiological response and is linked to the direct therapeutic effect of the modality (16,76). The advantages of using ORR as the end-point include that ORR is not affected by the natural history of the disease and it remains the fastest criterion to assess response in early phase II clinical trials. However, as discussed previously, imaging results are fraught with confounding effects of treatment itself, as well as the inherent differences in tumor enhancement characteristics. Furthermore, some agents, including targeted therapies, have the intended effect of achieving tumor control rather than regression. Isolated imaging-based metrics for outcomes would be biased against such therapies, which may otherwise have significant value. Blinded centralized radiologic review is a useful strategy to address these methodological issues including bias, but may not be feasible or cost-effective for phase II trials. This highlights the need for a multi-modal assessment of outcome.
OS is most frequently used as an end-point in Neuro-Oncology practice and clinical trials and is often considered as gold standard (76) as measurements are simple and precise. OS as an end-point is particularly useful in patients with PD such as GBM with relatively rapid recurrence and shorter survival time. Improvement in OS in such patients can be attributed to the therapeutic efficacy; however, this end-point is often affected by various concomitantly used salvage therapies and therefore need to be cautiously interpreted. Also variability in terms of inclusion criteria, performance level of patients during enrollment, number and types of salvage therapies used made comparison with historical controls using OS as end point challenging (16). Therefore to circumvent these issues, using OS at a defined time period along with standardizing various salvage therapies made it possible to compare OS with historical controls. However, PFS is usually preferred for such comparisons. PFS has several advantages over OS, including an attenuated time to event, potentially larger effect sizes, and outcomes independent of salvage therapy use (16,76). While PFS is not clearly a surrogate marker of OS, PFS at fixed time points (such as PFS 6 months) correlates well with OS based on previous studies (77-79). Despite this, the definition of PFS depends once again on imaging-based outcomes, lending it to the same inherent issues as previously outlined.
Current state of RANO in the assessment of brain tumor response
RANO for measuring response of high-grade gliomas
Given the limitations of the above criteria, the development of the RANO criteria attempts to circumvent some of these issues by including metrics which address both “measurable” and “non-measurable” lesions, in a time frame that minimizes the influence of treatment related effects and steroids while emphasizing durable (>4 weeks) outcomes. In this manner, RANO builds upon the positive elements of prior criteria while expanding applicability to the complex assessment of GBM response.
The inclusion of “non-measurable” disease in the RANO criteria is a significant change from prior proposed criteria. A recent study assessed the RANO criteria using outcome data of patients with recurrent GBM from a prospective randomized phase II trial (AVF3708) to determine the influence of these added measurements on assessment of ORR and PFS compared to the old Macdonald criteria (80). In this study, the addition of T2/FLAIR assessment by RANO resulted in statistically significant reductions in median PFS (28%) and ORRs, with a sustained correlation between progression and survival. The T2/FLAIR assessment resulted in earlier detection of progression in a subgroup of patients leading to this reduction, with detection of at least 35% of patients with non-enhancing tumor progression that would not have met progression criteria under Macdonald (80).
One concern regarding the RANO criteria has been the use of 2D measurements over volumetric measurements. A recent study from the randomized controlled phase II BELOB trial evaluating bevacizumab, lomustine, and combination therapy studied 2D vs. volumetric methods for response assessment using OS as the primary endpoint. This study compared 2D RANO with assessment by contrast-enhancing volume, subtraction volume, contrast enhancing and FLAIR volume, and subtraction and FLAIR volume. At second follow-up, only results from the RANO criteria and combination contrast/FLAIR analysis were sufficiently reliable (power >80%). Volumetric methods, with or without subtraction, did not provide significant improvement as a prognostic marker in bevacizumab-treated patients. Notably, the 2D RANO assessment was performed by “raters with extensive clinical experience”, which was proposed by the authors as a possible reason for enhanced performance of the 2D method in comparison with prior studies (81).
Current challenges in assessing response to therapy in patients undergoing resection of recurrent tumors
A major challenge with the applicability of RANO criteria is the ability to differentiate true tumor progression from pseudo progression (treatment after-effects) due to lack of reliable imaging modalities. As per the RANO criteria, true tumor progression within 12 weeks following chemoradiation/RT is diagnosed only if there is obvious tumor seen on histopathology examination. There are questions regarding period of 12 weeks as pseudo progression can occur after 12 weeks as well, histopathology definition of unequivocal evidence and cases in which histopathology is negative for tumor also need to be defined. Intratumoral heterogeneity with a mixture of tumor and treatment effects coupled with lack of defined diagnostic criteria in patients with recurrent tumor creates significant challenges for neuropathologists. In addition, biopsy from different regions of the same tumor may show different pathology and therefore sampling of the appropriate portion of the tumor becomes highly relevant in the diagnosis. Incorporating immunohistochemical markers (cell proliferation, inflammation, gliosis, etc.) with histopathology analysis, and validating correlation between histopathology, radiographic and clinical outcome are steps forward in overcoming the challenges associated with diagnosis and management of patients with recurrent glioma. These challenging issues are likely to be addressed with increasing experience of RANO criteria in future clinical trials.
Summary
Availability of effective and reliable criteria to assess response of high-grade glioma to various treatment modalities is critical in Neuro-Oncology practice not only to evaluate the role of currently available therapeutic options but also to assess and compare newer treatment modalities across different clinical trials. The guidelines laid down by the RANO working group opened the horizons for exploring novel response assessment tools including imaging techniques. The RANO committee is actively working to incorporate standardized neurological and functional assessment scales into the standard RANO criteria, to integrate imaging, clinical and functional response measures. Future RANO versions shall incorporate some of the rapid developments in this area towards a uniform and standardized assessment scheme, which can be integrated in future clinical trials. Various newly introduced RANO criterions shall undergo further refining and modification in the process of standardization, as these criteria are incorporated in future clinical trials. RANO is also in the process of developing standardized criteria to assess response in patients with leptomeningeal disease, meningioma and a variety of pediatric brain tumors.
Acknowledgements
None.
Footnote
Conflicts of Interest: MA Vogelbaum is co-founder and a member of the steering committee of RANO. He receives no compensation for this activity. MA Vogelbaum is co-founder of Infuseon Therapeutics and has patent interests in drug delivery devices licensed to Infuseon. The other authors have no conflicts of interest to declare.
References
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Gilbert MR. Recurrent glioblastoma: a fresh look at current therapies and emerging novel approaches. Semin Oncol 2011;38 Suppl 4:S21-33. [Crossref] [PubMed]
- Norden AD, Drappatz J, Muzikansky A, et al. An exploratory survival analysis of anti-angiogenic therapy for recurrent malignant glioma. J Neurooncol 2009;92:149-55. [Crossref] [PubMed]
- Leu K, Pope WB, Cloughesy TF, et al. Imaging biomarkers for antiangiogenic therapy in malignant gliomas. CNS Oncol 2013;2:33-47. [Crossref] [PubMed]
- Levin VA, Crafts DC, Norman DM, et al. Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg 1977;47:329-35. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Macdonald DR, Cascino TL, Schold SC Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8:1277-80. [Crossref] [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010;28:1963-72. [Crossref] [PubMed]
- Brandsma D, Stalpers L, Taal W, et al. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 2008;9:453-61. [Crossref] [PubMed]
- Cairncross JG, Pexman JH, Rathbone MP. Post-surgical contrast enhancement mimicking residual brain tumour. Can J Neurol Sci 1985;12:75. [Crossref] [PubMed]
- Cairncross JG, Pexman JH, Rathbone MP, et al. Postoperative contrast enhancement in patients with brain tumor. Ann Neurol 1985;17:570-2. [Crossref] [PubMed]
- Henegar MM, Moran CJ, Silbergeld DL. Early postoperative magnetic resonance imaging following nonneoplastic cortical resection. J Neurosurg 1996;84:174-9. [Crossref] [PubMed]
- Ulmer S, Braga TA, Barker FG 2nd, et al. Clinical and radiographic features of peritumoral infarction following resection of glioblastoma. Neurology 2006;67:1668-70. [Crossref] [PubMed]
- Galanis E, Wu W, Cloughesy T, et al. Phase 2 trial design in neuro-oncology revisited: a report from the RANO group. Lancet Oncol 2012;13:e196-204. [Crossref] [PubMed]
- Reardon DA, Galanis E, DeGroot JF, et al. Clinical trial end points for high-grade glioma: the evolving landscape. Neuro Oncol 2011;13:353-61. [Crossref] [PubMed]
- Lin NU, Lee EQ, Aoyama H, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 1: patient population, response, and progression. A report from the RANO group. Lancet Oncol 2013;14:e396-406. [Crossref] [PubMed]
- Lin NU, Wefel JS, Lee EQ, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. Lancet Oncol 2013;14:e407-16. [Crossref] [PubMed]
- van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol 2011;12:583-93. [Crossref] [PubMed]
- Chinot OL, Macdonald DR, Abrey LE, et al. Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep 2013;13:347. [Crossref] [PubMed]
- van den Bent MJ, Vogelbaum MA, Wen PY, et al. End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's Criteria. J Clin Oncol 2009;27:2905-8. [Crossref] [PubMed]
- Vogelbaum MA, Jost S, Aghi MK, et al. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery 2012;70:234-43; discussion 43-4. [Crossref] [PubMed]
- Westphal M, Ylä-Herttuala S, Martin J, et al. Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol 2013;14:823-33. [Crossref] [PubMed]
- Linhares P, Carvalho B, Figueiredo R, et al. Early Pseudoprogression following Chemoradiotherapy in Glioblastoma Patients: The Value of RANO Evaluation. J Oncol 2013;2013:690585.
- Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 2008;26:2192-7. [Crossref] [PubMed]
- Brandes AA, Tosoni A, Spagnolli F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in Neuro-Oncology. Neuro Oncol 2008;10:361-7. [Crossref] [PubMed]
- Chaskis C, Neyns B, Michotte A, et al. Pseudoprogression after radiotherapy with concurrent temozolomide for high-grade glioma: clinical observations and working recommendations. Surg Neurol 2009;72:423-8. [Crossref] [PubMed]
- Nasseri M, Gahramanov S, Netto JP, et al. Evaluation of pseudoprogression in patients with glioblastoma multiforme using dynamic magnetic resonance imaging with ferumoxytol calls RANO criteria into question. Neuro Oncol 2014;16:1146-54. [Crossref] [PubMed]
- Gallego Perez-Larraya J, Lahutte M, Petrirena G, et al. Response assessment in recurrent glioblastoma treated with irinotecan-bevacizumab: comparative analysis of the Macdonald, RECIST, RANO, and RECIST + F criteria. Neuro Oncol 2012;14:667-73. [Crossref] [PubMed]
- Galanis E, Buckner JC, Maurer MJ, et al. Validation of neuroradiologic response assessment in gliomas: measurement by RECIST, two-dimensional, computer-assisted tumor area, and computer-assisted tumor volume methods. Neuro Oncol 2006;8:156-65. [Crossref] [PubMed]
- Shah GD, Kesari S, Xu R, et al. Comparison of linear and volumetric criteria in assessing tumor response in adult high-grade gliomas. Neuro Oncol 2006;8:38-46. [Crossref] [PubMed]
- Kanaly CW, Mehta AI, Ding D, et al. A novel, reproducible, and objective method for volumetric magnetic resonance imaging assessment of enhancing glioblastoma. J Neurosurg 2014;121:536-42. [Crossref] [PubMed]
- de Groot JF, Mandel JJ. Update on anti-angiogenic treatment for malignant gliomas. Curr Oncol Rep 2014;16:380. [Crossref] [PubMed]
- Reuter M, Gerstner ER, Rapalino O, et al. Impact of MRI head placement on glioma response assessment. J Neurooncol 2014;118:123-9. [Crossref] [PubMed]
- Chi AS, Sorensen AG, Jain RK, et al. Angiogenesis as a therapeutic target in malignant gliomas. Oncologist 2009;14:621-36. [Crossref] [PubMed]
- Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol 2009;22:633-8. [Crossref] [PubMed]
- Clarke JL, Chang S. Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr Neurol Neurosci Rep 2009;9:241-6. [Crossref] [PubMed]
- Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733-40. [Crossref] [PubMed]
- Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 2009;27:740-5. [Crossref] [PubMed]
- Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 2008;70:779-87. [Crossref] [PubMed]
- Zuniga RM, Torcuator R, Jain R, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol 2009;91:329-36. [Crossref] [PubMed]
- Pinho MC, Polaskova P, Kalpathy-Cramer J, et al. Low incidence of pseudoprogression by imaging in newly diagnosed glioblastoma patients treated with cediranib in combination with chemoradiation. Oncologist 2014;19:75-81. [Crossref] [PubMed]
- Chamberlain MC. Radiographic patterns of relapse in glioblastoma. J Neurooncol 2011;101:319-23. [Crossref] [PubMed]
- Wick W, Wick A, Weiler M, et al. Patterns of progression in malignant glioma following anti-VEGF therapy: perceptions and evidence. Curr Neurol Neurosci Rep 2011;11:305-12. [Crossref] [PubMed]
- de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol 2010;12:233-42. [Crossref] [PubMed]
- Gerstner ER, Chen PJ, Wen PY, et al. Infiltrative patterns of glioblastoma spread detected via diffusion MRI after treatment with cediranib. Neuro Oncol 2010;12:466-72. [PubMed]
- Pope WB, Young JR, Ellingson BM. Advances in MRI assessment of gliomas and response to anti-VEGF therapy. Curr Neurol Neurosci Rep 2011;11:336-44. [Crossref] [PubMed]
- Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 2014;15:943-53. [Crossref] [PubMed]
- Ananthnarayan S, Bahng J, Roring J, et al. Time course of imaging changes of GBM during extended bevacizumab treatment. J Neurooncol 2008;88:339-47. [Crossref] [PubMed]
- Pope WB, Sayre J, Perlina A, et al. MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol 2005;26:2466-74. [PubMed]
- Quant EC, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol 2009;11:550-5. [Crossref] [PubMed]
- Wick A, Dorner N, Schafer N, et al. Bevacizumab does not increase the risk of remote relapse in malignant glioma. Ann Neurol 2011;69:586-92. [Crossref] [PubMed]
- Boxerman JL, Zhang Z, Safriel Y, et al. Early post-bevacizumab progression on contrast-enhanced MRI as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 Central Reader Study. Neuro Oncol 2013;15:945-54. [Crossref] [PubMed]
- Artzi M, Bokstein F, Blumenthal DT, et al. Differentiation between vasogenic-edema versus tumor-infiltrative area in patients with glioblastoma during bevacizumab therapy: a longitudinal MRI study. Eur J Radiol 2014;83:1250-6. [Crossref] [PubMed]
- Hattingen E, Jurcoane A, Daneshvar K, et al. Quantitative T2 mapping of recurrent glioblastoma under bevacizumab improves monitoring for non-enhancing tumor progression and predicts overall survival. Neuro Oncol 2013;15:1395-404. [Crossref] [PubMed]
- Ellingson BM, Cloughesy TF, Lai A, et al. Quantification of edema reduction using differential quantitative T2 (DQT2) relaxometry mapping in recurrent glioblastoma treated with bevacizumab. J Neurooncol 2012;106:111-9. [Crossref] [PubMed]
- Hoehn-Berlage M, Tolxdorff T, Bockhorst K, et al. In vivo NMR T2 relaxation of experimental brain tumors in the cat: a multiparameter tissue characterization. Magn Reson Imaging 1992;10:935-47. [Crossref] [PubMed]
- Oh J, Cha S, Aiken AH, et al. Quantitative apparent diffusion coefficients and T2 relaxation times in characterizing contrast enhancing brain tumors and regions of peritumoral edema. J Magn Reson Imaging 2005;21:701-8. [Crossref] [PubMed]
- Schwarzenberg J, Czernin J, Cloughesy TF, et al. 3'-deoxy-3'-18F-fluorothymidine PET and MRI for early survival predictions in patients with recurrent malignant glioma treated with bevacizumab. J Nucl Med 2012;53:29-36. [Crossref] [PubMed]
- Harris RJ, Cloughesy TF, Pope WB, et al. 18F-FDOPA and 18F-FLT positron emission tomography parametric response maps predict response in recurrent malignant gliomas treated with bevacizumab. Neuro Oncol 2012;14:1079-89. [Crossref] [PubMed]
- Albert NL, Weller M, Suchorska B, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol 2016;18:1199-208. [Crossref] [PubMed]
- Ellingson BM, Bendszus M, Boxerman J, et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol 2015;17:1188-98. [PubMed]
- Correia MM, Carpenter TA, Williams GB. Looking for the optimal DTI acquisition scheme given a maximum scan time: are more b-values a waste of time? Magn Reson Imaging 2009;27:163-75. [Crossref] [PubMed]
- Grech-Sollars M, Hales PW, Miyazaki K, et al. Multi-centre reproducibility of diffusion MRI parameters for clinical sequences in the brain. NMR Biomed 2015;28:468-85. [Crossref] [PubMed]
- Heiss JD, Papavassiliou E, Merrill MJ, et al. Mechanism of dexamethasone suppression of brain tumor-associated vascular permeability in rats. Involvement of the glucocorticoid receptor and vascular permeability factor. J Clin Invest 1996;98:1400-8. [Crossref] [PubMed]
- Piette C, Deprez M, Roger T, et al. The dexamethasone-induced inhibition of proliferation, migration, and invasion in glioma cell lines is antagonized by macrophage migration inhibitory factor (MIF) and can be enhanced by specific MIF inhibitors. J Biol Chem 2009;284:32483-92. [Crossref] [PubMed]
- Vredenburgh JJ, Cloughesy T, Samant M, et al. Corticosteroid use in patients with glioblastoma at first or second relapse treated with bevacizumab in the BRAIN study. Oncologist 2010;15:1329-34. [Crossref] [PubMed]
- Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol 2013;31:3212-8. [Crossref] [PubMed]
- Pasieka JL. Dare to be Oslerian: embracing the value of clinical medicine in today's surgical practice. Surgery 2010;148:1047-56. [Crossref] [PubMed]
- Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol 2006;24:1305-9. [Crossref] [PubMed]
- Armstrong TS, Gilbert MR. Net clinical benefit: functional endpoints in brain tumor clinical trials. Curr Oncol Rep 2007;9:60-5. [Crossref] [PubMed]
- Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia 2010;51:676-85. [Crossref] [PubMed]
- Wieser HG, Blume WT, Fish D, et al. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia 2001;42:282-6. [Crossref] [PubMed]
- Avila EK, Chamberlain M, Schiff D, et al. Seizure control as a new metric in assessing efficacy of tumor treatment in low-grade glioma trials. Neuro Oncol 2017;19:12-21. [Crossref] [PubMed]
- Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 2010;19:539-49. [Crossref] [PubMed]
- Alexander BM, Galanis E, Yung WK, et al. Brain Malignancy Steering Committee clinical trials planning workshop: report from the Targeted Therapies Working Group. Neuro Oncol 2015;17:180-8. [Crossref] [PubMed]
- Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol 2007;9:29-38. [Crossref] [PubMed]
- Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 2008;10:162-70. [Crossref] [PubMed]
- Han K, Ren M, Wick W, et al. Progression-free survival as a surrogate endpoint for overall survival in glioblastoma: a literature-based meta-analysis from 91 trials. Neuro Oncol 2014;16:696-706. [Crossref] [PubMed]
- Huang RY, Rahman R, Ballman KV, et al. The Impact of T2/FLAIR Evaluation per RANO Criteria on Response Assessment of Recurrent Glioblastoma Patients Treated with Bevacizumab. Clin Cancer Res 2016;22:575-81. [Crossref] [PubMed]
- Gahrmann R, van den Bent M, van der Holt B, et al. Comparison of 2D (RANO) and volumetric methods for assessment of recurrent glioblastoma treated with bevacizumab-a report from the BELOB trial. Neuro Oncol 2017;19:853-61. [Crossref] [PubMed]


