Pancreas adenocarcinoma: novel therapeutics
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the third highest cause of cancer-related deaths in the US, and is projected to be second only to non-small cell lung cancer (NSCLC) by the 2020s (1). In 2017, there are estimated to be 53,670 new cases of pancreas cancer and 43,090 deaths (2). Of these, 85% will present with unresectable disease, with a 5-year survival of 3% in patients who are metastatic at diagnosis (3). Despite these challenging figures, exciting novel therapeutics are in development and there is hope for improved outcomes in the near future.
Current therapies for metastatic PDAC
For decades, gemcitabine was a standard of care for first line treatment of unresectable and metastatic PDAC (4). In 2007, erlotinib with gemcitabine was approved, but more recently, FOLFIRINOX (folinic acid, 5-FU, irinotecan and oxaliplatin) and gemcitabine with nab-paclitaxel (GN) have become the two upfront standards of care regimens. FOLFIRINOX demonstrated a median overall survival (mOS) of 11.2 vs. 6.8 months for gemcitabine (P=0.001) (PRODIGE-III-2011) (5). GN improved mOS from 6.7 to 8.5 months (P=0.001) compared to gemcitabine (MPACT-2013) (6). Recently, as second line and beyond, nano-liposomal irinotecan in combination with 5-FU and leucovorin was approved in patients previously treated with gemcitabine (NAPOLI-1-2015). Nano-liposomal irinotecan with 5-FU had a mOS of 6.1 months compared to 4.2 months for 5-FU and leucovorin alone (P=0.012) (7).
Despite studying numerous agents over the last decade, almost none beyond cytotoxic agents have demonstrated survival benefit in late stage PDAC studies. Nonetheless, our understanding of PDAC pathobiology has evolved, including genetic basis, microenvironment and immune response.
Pathobiology
Over 90% of PDAC’s are driven by early activating KRAS mutations (8,9). Activated KRAS signals through a phosphorylation cascade of RAF, MEK and ERK lead to transcription of proliferation genes. In parallel, RAS activation increases signaling through PI3K, AKT and mTOR also lead to transcription of pro-survival and proliferation genes (10). Inactivating mutations and deletions in genes that regulate the cell cycle are frequent in PDAC. In a set of 336 patients whose tumors underwent genomic profiling at Memorial Sloan Kettering Cancer Center, mutations in TP53 were present in 72% and CDKN2A in 18% with 9p21 deletions involving CDKN2A and/or CDKN2B in 14%. TGF-βeta effector SMAD4 was mutated in 22%. Beyond these alterations, of the 256 different mutated genes and 128 different genes with copy number alterations, only ARID1A was altered in >10% of PDACs, although 4.2% of these patients had germline mutations in BRCA2 (9). These patients with germline DNA repair deficits represent an important subset with physiology that appears to predispose sensitivity to DNA damaging agents.
Histologically, PDAC is characterized by a dense stroma composing an average of 48% of tumor volume in one series (11). Stromal components include fibroblasts, hyaluronic acid (HA), collagen and other extracellular matrix proteins, inflammatory cells and cancer stem cells (CSCs). There is debate whether the stroma supports malignancy or acts as a protective barrier, however, evidence supports the stroma impairing drug delivery and supporting an immunosuppressive tumor microenvironment (TME) (12). Although PDAC is not considered an immunogenic cancer, activated CD8+ T cells are seen in the TME, but are far outnumbered by immunosuppressive inflammatory cells not seen in the normal pancreas (13). Novel therapeutics are in development targeting the stroma to improve drug delivery and the immune response. Stromal modifying therapy and immunotherapeutics as well as novel targeted agents and metabolism-directed strategies will be reviewed in detail. A selection of ongoing trials are described in Table 1 and therapeutic targets are depicted in Figure 1.
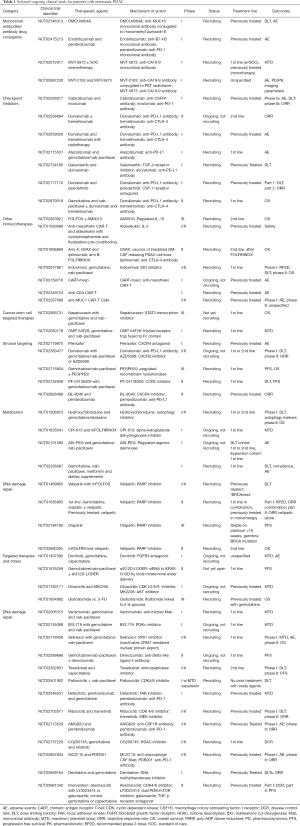
Full table
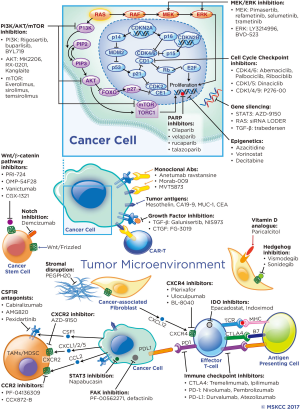
Stromal targeting agents
Enzymatic stromal disruption strategies
The PDAC stroma contains an abundance of hyaluronan (HA), collagen and fibronectin, which are deposited by activated cancer-associated fibroblasts (CAFs). HA forms a gel-like, compression resistant fluid leading to increased interstitial pressures causing vessel collapse, impeding blood flow and reducing therapeutic delivery in murine PDAC models (14). A retrospective study evaluating HA levels in human PDAC found that high HA tumor staining was associated with worse overall survival (mOS 9.3 vs. 24.3 months; P=0.037) (15).
PEGPH20 is a recombinant pegylated hyaluronidase enzyme developed to target tumor stromal HA effects. In a study using KPC (KrasLSL.G12D/+; p53R172H/+; PdxCretg/+) mice, PEGPH20 administration improved vessel patency and increased intra-tumoral delivery of doxorubicin and gemcitabine. Furthermore, combination therapy with gemcitabine and PEGPH20 led to improved survival relative to gemcitabine alone (28.5 vs. 15 days; P=0.002) (16).
Early stage studies of PEGPH20 have shown promise. A phase Ib non-randomized single arm study of PEGPH20 in combination with gemcitabine as first line treatment for metastatic PDAC demonstrated a signal of interest in patients with high HA levels when compared to the low HA groups with median progression free survival (mPFS) of 7.2 vs. 3.5 months and mOS of 13 vs. 5.7 months (17).
Randomized phase II results of PEGPH20 in combination with GN compared to GN continue to demonstrate potential benefit, notably and perhaps exclusively in high HA patients. Utilizing a tissue based immunohistochemistry assay to determine HA levels, the study met the pre-specified secondary endpoint of mPFS in the high HA group (9.2 vs. 5.2 months; P=0.048). The study underwent a temporary hold due to increased thrombosis rate, both venous and arterial, in the PEGPH20 arm (43% vs. 25%). After resumption of the trial (stage II), high risk patients for thromboembolism were excluded and all patients were placed on low molecular weight heparin primary prophylaxis resulting in similar thrombosis rates between groups and mitigating the negative signal. It is notable, that many patients discontinued treatment during the clinical hold potentially decreasing the treatment effect in stage I analysis. In stage II (following hold and with institution of low molecular weight heparin), an improved PFS signal was observed in high HA patients and a trend towards improved OS was seen in the high HA group 11.7 vs. 7.8 months (HR 0.52, 0.22–1.23) (18).
Recently, however, a phase I/randomized phase II of first line mFOLFIRINOX ± PEGPH20 (NCT01959139) in a non-biomarker selected population was stopped for futility at interim analysis (Table 2). The data set is being further analyzed including a retrospective analysis of tumor HA levels (25).
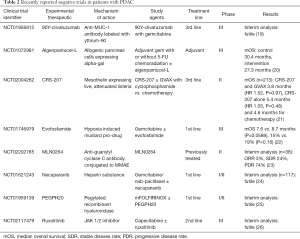
Full table
A phase III registration study for first line PEGPH20 in combination with GN vs. GN and placebo is recruiting in HA high patients (NCT02715804) and our group is evaluating PEGPH20 and GN in patients at higher risk for thrombosis in combination with rivaroxaban (NCT02921022).
Chemokines and the immune stroma
The immunosuppressive nature of the PDAC TME is established early in disease with interstitial aggregates of tumor-associated macrophages (TAMs), myeloid derived suppressor cells (MDSCs) and Tregs recruited from the bone marrow (13,27). Once in the microenvironment, MDSCs and TAMs inhibit proliferation and induce apoptosis of activated T-cells (27). The relatively small numbers of cytotoxic T cells in the microenvironment are rarely found in proximity to tumor epithelial cells and have down regulation of the activating T-cell co-receptor CD3 (28,29). An emerging strategy to combat the immunosuppressive TME targets the chemokine receptors that mobilize immunosuppressive cells and inhibit migration of effector cells.
CXCR4 is a chemokine receptor expressed by effector T-lymphocytes. In the TME, the CXCR4 ligand, CXCL12, is secreted by fibroblast activation protein-alpha positive CAFs (FAP+ CAFs). In a KPC mouse model, CXCL12, coated malignant cells and appeared to exclude effector T-cells from regions with tumor epithelial cells. Depletion of CAFs or administration of the CXCR4 inhibitor, plerixafor, induced the accumulation of tumor-infiltrating lymphocytes among cancer cells and acted synergistically with PDL-1 inhibition (30). CXCR4 has also been identified on PDAC cells and PDAC stem cells as a mediator of invasion and engraftment in the pre-metastatic niche (31,32). Plerixafor is currently being evaluated in a phase I dose finding study (NCT02179970). BL-8040, a peptide inhibitor of CXCR4, is under investigation in two phase II studies in combination with pembrolizumab (NCT02907099, NCT02826486). And, ulocuplumab, an anti-CXCR4 monoclonal antibody, has been studied in combination with nivolumab in a phase I/II of metastatic PDAC and NSCLC (NCT02472977) and results are awaited.
CXCR2 and CCR2 are chemokine receptors expressed on immunosuppressive inflammatory cells. CXCR2 on MDSCs and TAMs is stimulated by CXCL1, CXCL2 and CXCL5 secreted by tumor cells and FAP+ CAFs (33). In a murine model, inhibition of CXCR2 decreased the number of myeloid cells in primary tumors and decreased metastases through prevention of metastatic niche formation (33). A phase I/II study of CXCR2 inhibitor, AZD5069, in combination with durvalumab is recruiting (NCT02583477).
CCR2 is expressed by macrophages with its ligand, CCL2, secreted by PDAC cells leading to macrophages infiltrating PDAC tumors (27). Blockade of CCR2 in mice with the novel agent, PF-04136309, decreased infiltration of inflammatory monocytes and TAMs in tumors and pre-metastatic liver and reduced tumor growth and metastases when combined with gemcitabine (27). PF-04136309 was evaluated in a phase Ib with expansion cohort in borderline resectable and locally advanced disease in combination with first line FOLFIRINOX. Sixteen of 33 patients who obtained repeat imaging had an objective response and 32 of 33 had disease control (34). A phase IB/randomized phase II study in combination with first line GN is currently underway (NCT02732938). CCR2 inhibitor, CCX872-B, has also demonstrated efficacy in an orthotopic mouse model decreasing tumor size by 42% and the proportion of m-MDSCs by 45% (35). In a mostly metastatic phase I population, CCX872-B in combination with FOLRINOX had an ORR of 37% with SD of 41%. Thirteen of 21 patients with available imaging at 24 weeks were progression free (36).
Macrophages colony stimulating factor-1 (CSF-1) is over-secreted by PDAC cells and is a crucial survival factor for TAMs (37). CSF-1 receptor (CSF-1R) inhibition leads to death of CSF-1 differentiated macrophages or functional reprogramming that enhances antigen presentation and T-cell response (38). In a murine PDAC model, CSF-1 inhibition also led to increased expression of PDL-1 and CTLA-4 and therapeutic synergy with anti-PD-1 and anti-CTLA4 inhibitors (37). Four CSF-1R inhibitors are under study in early phase trials in combination with PD-1/PD-L1 inhibitors: AMG820 in combination with pembrolizumab (NCT02713529), cabiralizumab with nivolumab (NCT02526017), pexidartinib with durvalumab (NCT02777710) and MCS110 in combination with PDR001 (NCT02807844).
Indoleamine 2,3-dioxygenase (IDO)-inhibition
The enzyme IDO catabolizes tryptophan into kynurenine which is toxic to effector T cells and leads to Treg differentiation (39). In cellular assays, IDO inhibition promotes T-cell and natural killer cell growth and reduces Treg cells (40). A phase I of IDO inhibitor, epacadostat, in combination with pembrolizumab in multiple malignancies recently completed (41). Indoximod has been studied in a phase I/II study as first line treatment in combination with GN (NCT02077881). At interim analysis 11/30 patients had an objective response including one complete response (42).
Hedgehog
The hedgehog pathway is involved in cancer stemness, stromal desmoplasia and various developmental pathways (43,44). Sonic hedgehog activity is increased in the stroma and believed to activate CAFs (44,45). There is mixed data as to whether targeting the hedgehog pathway and resultant stromal desmoplasia inhibits or promotes PDAC progression (46). Early studies of smoothened inhibitors, vismodegib, saridegib and sonidegib, failed to demonstrate improved survival compared to controls (47-49). Both sonidegib and vismodegib are under study in combination with GN (NCT01431794, NCT01064622). At ASCO 2014, interim results were presented for the vismodegib study showing mPFS of 5.5 months and OS 10 of months (50).
Vitamin D
Activated pancreatic stellate cells (PSCs) are important precursors to CAFs (51). Recent data has demonstrated that vitamin D receptor (VDR) agonism modulates PSC activation reversing lipid droplet loss and expression of alpha-smooth muscle actin (52). Furthermore, incubation of PDAC cell lines in culture media from calcipotriol treated cancer-associated PSCs (CAPSCs) decreases proliferative gene expression including CXCL1, CSF2 and phospho-STAT3 relative to CAPSC culture media alone. In vivo, KPC mice treated with gemcitabine and calcipotriol had tumor volume decrease (70%), intratumoral gemcitabine increase and a 57% increase in survival compared to chemotherapy alone (52). Vitamin D3 has also been reported to induce differentiation of immature myeloid cells and increases in intratumoral CD4+ and CD8+ T cells in NSCLC and head and neck cancers, which may offer another mechanism of benefit in PDAC (53).
Paricalcitol is currently being studied in a single arm phase II study in combination with nivolumab, gemcitabine, nab-paclitaxel and cisplatin for first line metastatic disease (NCT02754726) and a neoadjuvant pilot in which it will be administered during a single cycle of GN versus GN alone before surgery (NCT02754726). Adverse effects as well as cellular and imaging markers will be evaluated in the latter.
Focal adhesion kinase (FAK)
FAK is a cytoplasmic tyrosine kinase with multiple functions. FAK pathways promote motility, invasion, survival and epithelial to mesenchymal transition (54). In an orthotopic mouse model of PDAC, FAK inhibition led to fewer CAFs and macrophages in the TME, smaller tumor volume, less invasion and fewer metastasis compared to controls (55). Phase I studies of PF-00562271 and GSK2256098 in combination with trametinib have completed (NCT00666926, NCT01938443). A phase II of second line GSK2256098 with trametinib and a phase I of defactinib with pembrolizumab are recruiting (NCT02428270, NCT02546531).
CSCs
The CSC theory posits that carcinogenesis is initiated by a relatively small number of stem cells or early progenitor cells, which develop the capabilities of self-renewal and multi-lineage differentiation (56). In PDAC, stem cells are characterized by the expression of either CD44+/CD24+/ESA+ or CD133+ (± CXCR4+) surface markers and demonstrate resistance to gemcitabine and ionizing radiation (32,57,58).
Therapeutics aimed at these resistant cells target the Wnt/β-catenin, notch, hedgehog and JAK/STAT pathways.
Wnt/β-catenin
Aberrant activation of the Wnt pathway occurs in up to 65% of pancreatic cancer patients. Wnt pathway factors contribute to the epithelial-mesenchymal transition in PDAC and may be responsible for resistance to gemcitabine, DNA damage and ionizing radiation (59-61).
Inhibition of Wnt in a mouse model inhibits PanIN formation and OMP-54F8, a frizzled trap, decreased CSCs and reduced tumor growth in a patient derived xenograft greater than gemcitabine (62,63). OMP-54F8 is currently being studied in a phase I study with GN (NCT02050178). PRI-724 is a small molecule inhibitor of the Wnt target gene coactivator creb binding protein, and is the only wnt pathway inhibitor with clinical results in PDAC. In combination with gemcitabine as second line therapy, no responses were observed, but 62.5% of patients had a >30% reduction in CA19-9 and mPFS was 2 months (64). Two other wnt pathway inhibitors (CGX-1321 and Vantictumab) are in early phase clinical study (NCT02675946, NCT02005315).
Notch
Notch signaling is crucial for the embryonic development of multiple tissues including the pancreas and is upregulated in early pancreatic carcinogenesis and invasive disease (65-68). Increased notch activity facilitates the EMT and gives rise to CD44 positive pancreatic CSCs (69). Pre-clinical studies of notch inhibition including patient derived xenograft models showed promising results, however, clinical development has been more sobering (70). Tarextumab, a monoclonal antibody against notch 2/3, showed no survival benefit in a phase II study with GN and possibly worse outcomes in low notch expressers (71). Gamma secretase inhibitor, RO4929097 has been discontinued, and a phase I of MK0752 with gemcitabine had severe GI adverse effects leading to high patient withdrawal (72,73). Anti-delta-like ligand 4 monoclonal antibody, demcizumab, continues to be studied in PDAC. In a phase I study in combination with first line GN, mPFS was 9.0 months and mOS 10.1 months, however, 2 patients developed reversible pulmonary hypertension, 1 of which developed heart failure, leading to abbreviated treatment dosing regimens of 70 days in the later cohorts (74). A randomized phase II study in combination with GN is completed and data is awaited. All arms received continuous cycles of GN until progression. One intervention arm received 3 cycles of demcizumab, the other received demcizumab for 3 cycles on, 3 cycles off and another 3 cycles on (NCT02289898).
JAK/STAT
Activation of JAK family receptors leads to phosphorylation of the STAT family transcription factors. JAK2/STAT3, in particular, is activated in PDAC with target genes including cyclin D1, p21, BCR-XL and VEGF contributing to cell cycle progression, apoptosis resistance and angiogenesis (75,76). In mice orthotopically injected with KRAS G12D shp53 cells, STAT3 knockdown leads to lower tumor volume and decreased proliferation than controls (77).
Early and mid-stage trials of JAK inhibitors showed promising initial results. In phase II, ruxolitinib and capecitabine vs. capecitabine and placebo demonstrated increased overall survival, 4.3 vs. 1.5 months, particularly in the high CRP subgroup (78). Ruxolitinib progressed to the phase III study, JANUS-1, where it again was in combination with capecitabine vs. capecitabine and placebo as second line treatment in patients with CRP >10. Unfortunately, as has been the case in a number of recent studies, interim analysis demonstrated futility (Table 2). Further development of ruxolitinib was discontinued in both the JANUS 1 and 2 trials (79).
Inhibition of STAT3 transcription by napabucasin has demonstrated ability to decrease relapse in PaCa-2 xenografts. Xenografts treated with gemcitabine or napabucasin for 41 days both exhibited decreased tumor growth compared to vehicle, however, when treatment was stopped tumors from gemcitabine treated mice continued to grow during the 22-day observation period, whereas napabucasin treated mice did not (80). Napabucasin has shown promising early clinical data (81). In a phase I/II in combination with paclitaxel in previously treated patients with a median of 2 prior regimens, a 52% disease control rate was achieved with mPFS of 10 weeks and mOS of 24 weeks (82). More recently in a phase Ib/II in combination with GN as first line treatment, the intention to treat population achieved a DCR of 77% and ORR of 38% with maturing mPFS and mOS of >7.4 and >10.4 months, respectively (83). A phase III trial of napabucasin in combination with GN vs. GN and placebo is planned (NCT02993731). AZD-9150 is an anti-sense STAT3 inhibitor with a planned phase II in combination with durvalumab in multiple malignancies (NCT02983578).
Targeted therapy
Beyond the approval of erlotinib with gemcitabine, targeted therapy has demonstrated little efficacy in PDAC. Most targeted therapy has been directed at the RAS pathway or epidermal/platelet derived growth factor receptors (EGFR/PDGFR). RAS is a key driver of PDAC, mutated in 90–95% and found in early PanIN lesions. It or its downstream effectors, RAF, MEK and ERK, are therefore logical targets for inhibition. The most common mutations in RAS cause a constitutive activation and strategies to inhibit this activation have failed (84). Targeting RAS by inhibiting translation using small-interfering RNAs is a novel strategy to decrease RAS activity. siRNA against the G12D mutant RAS RNA by local prolonged delivery, siG12D LODER (Local Drug EluteR), has demonstrated decreased growth of human pancreatic tumor cells in vivo and prolonged mouse survival (85). In a phase Ib/II dose escalation 15 locally advanced, unresectable patients received siG12D LODER with a mOS of 15.12 months. Ten out of 12 patients had SD on repeat imaging and 2 had a PR (86). siRNA LODER is planned to be evaluated in locally advanced disease by intratumoral injection in a randomized phase II in combination with GN with/without LODER (NCT01676259).
MEK/ERK/MAPK, PI3K/AKT/mTOR
Given the challenges with targeting RAS, the downstream effectors MEK and ERK offer alternatives with therapeutics in development. MEK and ERK inhibitors when used as monotherapy or in combination with chemotherapy have not demonstrated benefit (87-90). MEK inhibition can lead to increased activation of PI3/AKT through hyperactivation of EGFR (91). As a result, various combination strategies have been attempted combining MEK inhibitors with PI3K, AKT and EGFR inhibitors. Unfortunately, this strategy has not seen improved outcomes and in some cases increased toxicity (92-94).
There are promising novel targets that intersect with RAS pathways including cell-cycle checkpoint inhibitors and novel kinase receptors.
Cell-cycle inhibition
Cell cycle dysregulation is a hallmark of malignancy. Cell cycle checkpoints are controlled by interactions between cyclins and corresponding cyclin dependent kinases (CDKs). p53 and CDKN2 gene mutations are common in PDAC and their protein products are intimately tied to the regulation of cyclin-D/CDK4/6 and cyclin E/CDK2 complexes which are required for progression through the G1/S checkpoint (95-97). Pre-clinical studies of multi-CDK inhibitor, daniciclib, reduced subcutaneous growth in 100% of human pancreatic cancer xenografts and combination with gemcitabine was more effective than either agent alone (98). In vitro, dual inhibition of CDK4/6 and PI3K/MTOR has shown synergistic effects in suppression of the cell cycle and CDK4/6 and MEK inhibition was additive (99).
Multiple CDK inhibitors are being studied in metastatic PDAC. Palbociclib is a CDK4/6 inhibitor undergoing investigation in two phase I studies—in combination with nab-paclitaxel (NCT02501902) and in combination with carboplatin or cisplatin (NCT02897375). Ribociclib and abemaciclib are in clinical study in combination with MEK and PI3K/AKT/MTOR inhibitors. Ribociclib is being evaluated in phase I/II studies in combination with everolimus (NCT02985125) and in combination with trametinib (NCT02703571) both in previously treated PDAC. Abemaciclib is being evaluated in a 4-arm phase II trial alone, in combination with PI3K/MTOR inhibitor, LY3023414, or in combination with TGF inhibitor, galunisertib (NCT02981342).
TGF-beta
Early in oncogenesis TGF-β is cytostatic and pro-apoptotic, however, later in disease TGF-β pathway activity leads to proliferation and invasiveness (100). TGF-β pathway signals through multiple SMAD proteins regulating transcription of various genes involved in cell cycle regulation and differentiation (101). SMAD4 is commonly mutated in PDAC and TGF-β stimulation of homozygous deleted SMAD4 cells leads to activation of pro-invasive and anti-apoptotic pathways including upregulation of ERK, β-catenin and p-BAD (102). In a phase I/II study in previously treated patients, trabedersen, an antisense oligonucleotide of TGF-β2, achieved a mOS of 13.4 months for patients administered the planned phase II dosing (n=9; 95% CI: 2.2–39.7) (103). Galunisertib, a small molecule TGF-βR1 inhibitor, achieved a mOS of 9.1 months in combination with gemcitabine vs. 7.59 months for gemcitabine and placebo in a recent phase II study (104). Another phase II study is planned in combination with cell cycle inhibitor, abemaciclib, as described previously, while a phase I in combination with durvalumab is recruiting (NCT02981342, NCT02734160).
Epigenetics
Epigenetic modification is a crucial method of gene control in malignancy. In PDAC, histone acetylation changes promote resistance to oxidative stress, poorer differentiation and upregulation of DNA repair genes leading to chemotherapy resistance (105). HDAC inhibitor, azacitidine, is being evaluated in a phase II study after adjuvant GN in patients with node positive disease or persistently elevated CA19-9 (NCT01845805) and a phase I study of refractory, metastatic patients in combination with nab-paclitaxel. CG200745 is in phase I/II in combination with gemcitabine and erlotinib (NCT02737228). In pre-clinical studies, CG200745 in combination with gemcitabine and erlotinib inhibited growth of xenografts and overcame gemcitabine resistance in cell lines (106). DNA methyltransferase inhibitor, decitabine is being evaluated in 3 early stage studies in combination with gemcitabine or tetrahydrouracil (NCT02959164, NCT02847000, NCT02685228).
Immunotherapy
Immunotherapy is revolutionizing cancer therapy, although impacts in PDAC remain to be realized. Various methods to utilize the immune system to target cancers are being developed with promising results in other malignancies. Immune checkpoint inhibition in melanoma and NSCLC has achieved durable responses in approximately 20–25% of patients (107,108). Chimeric antigen receptor T cells, in which a patient’s own T-cells are collected, genetically altered to express a tumor antigen specific receptor and re-infused, are achieving complete responses in refractory leukemias and lymphomas (109-111). These modalities are emerging as potential treatment options in PDAC as well.
Checkpoint inhibition
Immune checkpoints consist of interactions between a series of surface proteins and their ligands to differentiate self from non-self. The classic model of effector cell stimulation involves an interaction between the MHC-presented antigen and the T-cell receptor and a costimulatory interaction between CD28 on T cells and B7 on antigen presenting cells (APCs). Additional inhibitory stimuli including PD-L1/PD-1 and CTLA-4/B7-1/2 have been identified and inhibitors developed. The PD-1 receptor is expressed on activated T cells, B cells and myeloid cells and its ligand PD-L1, is expressed on many cancers including PDAC. CTLA-4 is upregulated on activated CD4+, CD8+ and T-regulatory cells and interacts with B7 antigens on APCs (112-114). In a series of resected PDAC specimens, expression of B7-1, B7-2, PD-L1 on PDAC cells and CTLA-4 and PD-1 on tumor infiltrating immune cells were increased in both frequency (all >95%) and degree of expression (6.5 fold for CTLA-4 and 6.1 for PD-1) compared to normal control pancreas tissue. High PD-L1 expression in this series correlated with decreased survival (24 vs. 10 months, P<0.0001) (115).
Despite excellent results in other malignancies and pre-clinical rationale in PDAC, initial clinical studies with CTLA-4 inhibitors either alone or in combination with traditional chemotherapy have had modest results. A phase II of ipilimumab administered to 20 metastatic and 7 locally advanced patients for 2 cycles did not demonstrate any responses by traditional criteria, although one patient did have a delayed response after initial progression (116). Ipilimumab and tremelimumab have both been studied in combination with gemcitabine in phase I studies with responses in 2/16 and 2/34 patients and mOS of 8.5 and 7.4 months, respectively (117,118).
Anti-PD-1 therapy has met similar challenges in a phase I with 0/14 PDAC patients demonstrating a response, whereas anti-PD-L1, durvalumab, has demonstrated only a 7% response rate (119-121). Multiple PD-1 and PDL-1 inhibitors are in early-mid phase studies alone or in combination with chemotherapy but without reported results to this point. PD-1 inhibitor, nivolumab, is in a phase II as first line with GN, cisplatin and paricalcitol (NCT02988960) and as second line with nab-paclitaxel ± gemcitabine (NCT02309177). Pembrolizumab, PD-1 inhibitor, is in phase I/II studies as first or second line in combination with GN (NCT02331251) and mFOLFOX (NCT02331251). PDL-1 inhibitor, atezolizumab, is also being studied in a phase I in combination with GN (NCT02715531). It is noteworthy, that pembrolizumab has been approved for microsatellite instability-high and mismatch repair deficient cancers agnostic of tissue of origin (122) In PDAC specifically, a small sample of 4 mismatch repair deficient PDAC patients treated with pembrolizumab demonstrated 2 PRs and 2 SDs (123).
Due to the overall modest responses of single agent checkpoint inhibition and supporting pre-clinical evidence, the focus has shifted to combination approaches including dual checkpoint blockade, multi-modality immunotherapy, and combination with targeted therapeutics or radiation (RT). Durvalumab, and tremelimumab, are being studied in combination in phase II as first line treatment with GN versus GN (NCT02715531). They are also being studied as both first and second line treatment vs. durvalumab alone (NCT02527434, NCT02558894).
Dual modality immunotherapy has signaled benefit in a phase I study of ipilimumab and the cancer vaccine, GVAX, versus ipilimumab alone. mOS was 5.7 vs. 3.6 months (HR 0.51; P=0.072) and 1-year OS (27% vs. 7%). Additionally, 3/15 patients had prolonged disease stabilization of 31, 71 and 81 weeks (124). GVAX consists of genetically altered allogenic PDAC cells that express GM-CSF with the aim of inducing an immune response towards the PDAC cells. Multiple combination studies with GVAX are ongoing. GVAX and ipilimumab are being studied in a phase II study vs. FOLFIRINOX (NCT01896869). A phase I/II of GVAX with or without nivolumab in resectable disease and a phase II of GVAX with pembrolizumab and SBRT in locally advanced disease are both recruiting (NCT02451982, NCT02648282).
The combination of checkpoint inhibitors and chemokine inhibitors is a rational combination approach. Chemokine inhibitors aim to increase the number of effector cells in the PDAC stroma and checkpoint inhibitors aim to increase effector cell activity. Indeed, KPC mice treated with CXCR2 inhibition preceding PD-1 inhibition lived longer than mice treated with vehicle followed by PD-1 inhibition (33). Similarly, a mouse model of CXCR4/CXCL12 and PD-L1 inhibition demonstrated fewer malignant cells in mouse tumors than PD-L1 alone (30). As described above, CSF1R inhibition also improves response to checkpoint blockade in murine models (37). Combination studies with these agents were described previously.
The abscopal effect is a phenomenon in which tumors outside of the field of radiation are ‘treated’ by radiation. The theory is that radiation causes the expression of antigens and alteration in the TME enabling immune stimulation (125). Multiple studies are ongoing or in planning to evaluate dual checkpoint blockade and RT in the metastatic setting. Three phase I studies are recruiting or planned to evaluate various combinations of durvalumab, tremelimumab or both with RT (NCT02639026, NCT02311361, NCT02868632). Nivolumab and RT with or without ipilimumab are also recruiting (NCT02866383).
Chimeric antigen receptor T cells (CAR-T)
CAR-Ts are T cells that are genetically altered to express specific receptors for non-MHC moieties on cancer cells. When engaged, the receptor activates the T cell’s cytotoxic response. CAR-Ts have shown promising results in refractory leukemia and lymphomas, but have not yielded as promising results in solid malignancies (126). In PDAC, CAR-Ts directed at various targets are being evaluated. Anti-CEA CAR-Ts are being studied in patients with CEA positive cancers with liver metastases in combination with Y90 microspheres (NCT02416466, ongoing) and by hepatic artery infusion (NCT02850536, planned). Mesothelin is an attractive target for CAR-Ts as it is highly expressed on many malignancies including 85% of PDACs (127). Three phase I studies of CAR-Ts targeting mesothelin are ongoing, but not recruiting at the University of Pennsylvania (NCT02465983, NCT02159716, NCT01897415) and a single phase I/II enrolling patients with multiple mesothelin expressing malignancies is recruiting at the National Cancer Institute (NCT01583686). Studies evaluating CAR-Ts against MUC1 (NCT02587689), prostate stem cell antigen (NCT02744287) and NK receptors (NCT03018405) are recruiting, as well. Other targets in development for CAR-Ts in PDAC include Ca19-9 and MUC16.
Monoclonal antibodies
The surface targets of CAR-Ts have also seen the development of monoclonal antibodies and antibody drug conjugates. Anetumab ravtansine is an anti-mesothelin antibody conjugated to the tubulin inhibitor DM4. This has shown strong pre-clinical results eradicating tumor in 5/6 orthogenic mice models and significantly lowering tumor volume compared to gemcitabine in patient derived xenografts (128). A phase I dose finding study in ovarian cancer, mesothelioma and pancreatic cancer was performed, however, outcomes were not reported for PDAC (NCT01439152) (129). A phase II study in pancreatic cancer is planned. Morab-009 is an anti-mesothelin antibody that was studied in a phase II trial in combination with gemcitabine vs. gemcitabine alone (NCT00570713). No results have been published. SS1P is an anti-mesothelin antibody conjugated to pseudomonas toxin being studied in a phase I/II study of mesothelin expressing malignancies (NCT01362790).
MVT-5873 is an antibody targeting CA19-9. MVT-5873 has demonstrated cytotoxicity in murine xenograft models and is being studied in a phase I dose finding study evaluating MVT-5873 alone and in combination with GN for patients with CA19-9 positive malignancies (NCT00570713) (130). Preliminary data has shown acceptable toxicity with DLT of transient grade 3 AST, ALT and bilirubin elevations and potential efficacy with stable disease of >4 months in 24% of patients and Ca19-9 reductions of >50% in 48% of patients (131). MVT-5873 is being developed in parallel with MVT-2163, a radiolabeled anti-CA19-9 antibody designed as a PET imaging agent, which is in phase I in combination with MVT-5873 to evaluate pharmacokinetics and optimal timing of imaging (NCT02687230) and combined with lutetium as a radioisotope (MVT-1075; NCT03118349).
Combining immunotherapy and antibody dependent cellular cytotoxicity, enoblituzumab is an antibody with a high affinity Fc region designed to stimulate antibody dependent cytotoxicity when it binds the immune checkpoint molecule B7-H3 (132). It is being studied in phase I studies in combination with pembrolizumab (NCT02475213) and ipilimumab (NCT02381314).
Metabolism
Malignant cells commonly have different metabolic requirements than normal cells. Malignant cells tend to rely on glycolysis and anaerobic metabolism, require increased amount of glutamate and may be deficient in argininosuccinate synthase and asparagine synthetase (ASNS) (133-135). Autophagy is a key mechanism that PDAC utilizes to obtain glutamate and may be required for PDAC growth (136). Autophagy inhibitor, hydroxychloroquine, is being studied in a number of trials—as neoadjuvant therapy in a randomized trial of gemcitabine and nab-paclitaxel ± hydroxychloroquine (NCT01978184), as first or second line in combination with gemcitabine for metastatic disease (NCT01506973) and as monotherapy in previously treated metastatic patients (NCT01273805). CPI-613 inhibits mitochondrial metabolism by inhibiting alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase and has shown efficacy in PDAC xenografts and early phase investigation (137). In a phase I trial in combination with FOLFIRINOX, an ORR of 53.9% was found comparing favorably to 31.8% for FOLFIRINOX alone in the pivotal PRODIGE study (5,138). ADI-PEG 20 inhibits argininosuccinate synthase, the activity of which is required for arginine production in PDAC cells. A phase I/Ib study of ADI-PEG with GN demonstrated promising results with a mPFS of 6.1 months, mOS 11.3 months, 45% ORR and 91% tumor regression rate in patients treated with the recommended phase II dose in the first line setting (139). A randomized phase II/III trial is in development in untreated metastatic PDAC. Eryaspase, red blood cell encapsulated L-asparaginase, hydrolyses asparagine to aspartic acid leading to asparagine depletion and impaired protein synthesis in low ASNS expressing tumors. Interestingly, in a phase IIb second line study in combination with gemcitabine or FOLFOX, eryaspase treatment improved OS in both the low ASNS (HR 0.62, P value not specified) and entire study population (HR 0.57, P=0.034) (140).
DNA repair
A small but significant subset of PDAC patients has an inheritable genetic predisposition. In a case series of 175 patients with pancreatic cancer and suspicious personal or family history who were treated at Memorial Sloan Kettering Cancer Center, a pathogenic germline mutation was found in 15.1% (BRCA2 7.4%, BRCA1 2.2%, p16 1.1%, PALB2 0.6% and Lynch syndrome 2.2%) (141). In other series’ of high risk patients, ATM mutations have a prevalence of 1–5.7%. In an unselected group of PDAC patients the rate of BCRA1/2 mutations is between 3.6–7%, but is significantly higher in Ashkenazi Jewish patients than non-Ashkenazi Jewish patients (12.1% vs. 3.7%; P=0.05) (142,143).
A majority of identified inheritable genetic mutations in PDAC affect proteins involved in homologous recombination. Homologous recombination is the highest fidelity double stranded DNA repair mechanism and is also crucial for removing DNA crosslinks. The process is initiated when the MRN (MRE11–RAD50–NBS1) complex identifies and stabilizes a double stranded DNA break. The MRN complex activates ATM leading to a complex series of interactions that require BRCA1/2 interactions with RAD51 and PALB2 to complete DNA repair (144-146). Impaired homologous recombination leads to the use of alternative DNA repair mechanisms and the accumulation of mutations that increase the risk of carcinogenesis, but also confer an increased sensitivity to platinum agents. Platinum agents create DNA crosslinks, which require repair through HR. Indeed, a number of small case series have shown evidence that patients with BRCA-associated PDAC are responsive to platinum agents and a retrospective review of 71 patients with stage III and IV BRCA associated PDAC found improved survival in patients treated with platinum agents (22 vs. 9 months; P=0.039) (147-149).
Poly ADP ribose polymerase (PARP) is necessary for repair of single stranded DNA breaks, which if not repaired lead to double stranded breaks (150). Additionally, PARP is also involved in alternative double stranded DNA repair pathways in the absence of HR. In the setting of BRCA1/2 mutations, PARP inhibition leads to accumulation chromatid aberrations, cell cycle arrest and cell death (145). PARP inhibitor (PARPi) olaparib is approved in BRCA mutant ovarian cancer and in clinical development for PDAC. A phase II study of olaparib in germline BRCA (+) patients included 23 patients with PDAC and demonstrated a 22% response rate and 35% stable disease with 1 year survival of 41% (151). Olaparib is currently being evaluated in a phase III study for patients with germline BRCA mutations and metastatic disease who have had stable disease on platinum therapy for at least 16 weeks. Patients receive olaparib monotherapy maintenance or placebo after completing chemotherapy (NCT02184195). Olaparib is also being studied as second line therapy in a phase II study for patients with ‘BRCAness’—no BRCA mutation but a family history of BRCA associated cancers (NCT02677038) and a separate phase II for ‘BRCAness’ which also includes mutations in other DNA damage repair genes is being planned (NCT02511223).
The combination of platinum and PARP inhibitor is an attractive treatment regimen to exploit DNA damage repair deficits. Veliparib, is under evaluation in a 3-arm phase II study for patients with BRCA1/2 or PALB2 mutations in combination with first line gemcitabine/cisplatin vs. gemcitabine/cisplatin/placebo vs. veliparib alone (NCT01585805). A phase II evaluating second line mFOLFIRI with or without veliparib is recruiting without requirement for BRCA mutation or BRCAness (NCT02890355). A trial evaluating rucaparib as maintenance after induction platinum-based therapy in patients with deleterious germline or somatic BRCA mutations is planned.
Conclusions
Improving survival in PDAC is a critical unmet need. With increased understanding of PDAC pathobiology, novel therapeutics targeting the stroma, harnessing the power of the immune system and blocking aberrant signaling pathways have reached clinical development providing a sense of optimism that recent scientific advances will turn into clinical results. Beyond therapeutic discovery, trial design with strict success criteria is critical to expeditiously realizing these results. Rahib et al. investigated 32 phase III studies between 1997 and 2015, but found that half of studies with a paired phase II advanced to phase III despite a negative phase II outcome, contributing significantly to the overall 15% phase III success rate (152). Incorporating biomarkers and utilizing surrogate endpoints will also aid in maximizing therapeutic potential and identifying failing candidates early. Across multiple malignancies, Jardim et al. found that 57% of successful programs utilized biomarker driven patient selection, however, only 16% of failed drug programs did (153).
The application of these strategies is emerging in large collaborative trials. The Precision Promise trial is an initiative of the Pancreatic Cancer Action Network in which patients will undergo biomarker analysis with pathologic evaluation, genomic sequencing and transcriptome analysis to determine assignment in treatment arms focused on stromal disruption, DNA damage repair or immunotherapy. The trial is designed to be dynamic incorporating promising novel therapeutics and eliminating failing ones.
Still, despite optimism, with current approved treatment options, survival in PDAC remains limited, and it is recommended that all patients enroll in clinical trials with the hope, now more than ever, of improved outcomes from novel therapeutics.
Acknowledgements
David M. Rubenstein Center for Pancreatic Cancer Research. Eileen M. O’Reilly has received research funding from Celgene, Halozyme, BMS, MedImmune, Astra-Zenica, OncoMed, Momenta Pharmaceticals, Polaris, Roche and Genentech.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2017. Atlanta: American Cancer Society, 2017.
- Hernandez BY, Green MD, Cassel KD, et al. Preview of Hawaii Cancer Facts and Figures 2010. Hawaii Med J 2010;69:223-4. [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016;387:545-57. [Crossref] [PubMed]
- Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [Crossref] [PubMed]
- Lowery MA, Jordan EJ, Basturk O, et al. Real Time Genomic Profiling of Pancreatic Ductal Adenocarcinoma: Potential Actionability and Correlation with Clinical Phenotype. Clin Cancer Res 2017. In Press.
- Hezel AF, Kimmelman AC, Stanger BZ, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 2006;20:1218-49. [Crossref] [PubMed]
- Moffitt RA, Marayati R, Flate EL, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 2015;47:1168-78. [Crossref] [PubMed]
- Gore J, Korc M. Pancreatic cancer stroma: friend or foe? Cancer Cell 2014;25:711-2. [Crossref] [PubMed]
- Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 2007;67:9518-27. [Crossref] [PubMed]
- Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:418-29. [Crossref] [PubMed]
- Whatcott CJ, Diep CH, Jiang P, et al. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin Cancer Res 2015;21:3561-8. [Crossref] [PubMed]
- Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013;62:112-20. [Crossref] [PubMed]
- Hingorani SR, Harris WP, Beck JT, et al. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin Cancer Res 2016;22:2848-54. [Crossref] [PubMed]
- Study 202 Overall Results and Stage 2 Results (Investor Presentation), 1/5/2017. Available online: http://www.halozyme.com
- Immunomedics provides update on phase 3 pancrit-1 trial of clivatuzumab tetraxetan in patients with advanced pancreatic cancer (press release), 03/14/2016. Available online: http://www.immunomedics.com/
- NewLink Genetics Announces Results from Phase 3 IMPRESS Trial of Algenpantucel-L for Patients with Resected Pancreatic Cancer (press release), 05/09/2016. Available online: http://investors.linkp.com/releases.cfm
- Le DT, Ko AH, Wainberg ZA, et al. Results from a phase 2b, randomized, multicenter study of GVAX pancreas and CRS-207 compared to chemotherapy in adults with previously-treated metastatic pancreatic adenocarcinoma (mPDA) (ECLIPSE Study). J Clin Oncol 2017;35:suppl; abstr 345.
- Van Cutsem E, Lenz HJ, Furuse J, et al. Evofosfamide (TH-302) in combination with gemcitabine in previously untreated patients with metastatic or locally advanced unresectable pancreatic ductal adenocarcinoma: Primary analysis of the randomized, double-blind phase III MAESTRO study. Conference: ASCO GI 2016, San Francisco, CA, USA. J Clin Oncol 2016;34:suppl 4S; abstr 193.
- Almhanna K, Wright D, Mercadé TM, et al. Abstract CT117: A phase II trial of TAK-264, a novel antibody-drug conjugate (ADC), in patients with pancreatic adenocarcinoma expressing guanylyl cyclase C (GCC). AACR 107th Annual Meeting 2016; New Orleans, LA, USA.
- Momenta Discontinues Further Accrual of its Phase 2 Trial of Necuparanib in Patients with Pancreatic Cancer Following Planned Interim Futility Analysis (press release), 08/04/2016. Available online: http://ir.momentapharma.com/releases.cfm
- Halozyme Provides Update On SWOG Collaborative Group Clinical Study (press release), 3/30/2017. Available online: http://www.halozyme.com/newsroom/news-releases/default.aspx
- Incyte Announces Decision to Discontinue JANUS Studies of Ruxolitinib plus Capecitabine in Patients with Advanced or Metastatic Pancreatic Cancer (press release), 02/11/2016. Available online: http://www.incyte.com/ir/press-releases.aspx
- Sanford DE, Belt BA, Panni RZ, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res 2013;19:3404-15. [Crossref] [PubMed]
- Ademmer K, Ebert M, Muller-Ostermeyer F, et al. Effector T lymphocyte subsets in human pancreatic cancer: detection of CD8+CD18+ cells and CD8+CD103+ cells by multi-epitope imaging. Clin Exp Immunol 1998;112:21-6. [Crossref] [PubMed]
- von Bernstorff W, Voss M, Freichel S, et al. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res 2001;7:925s-32s. [PubMed]
- Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 2013;110:20212-7. [Crossref] [PubMed]
- Mori T, Doi R, Koizumi M, et al. CXCR4 antagonist inhibits stromal cell-derived factor 1-induced migration and invasion of human pancreatic cancer. Mol Cancer Ther 2004;3:29-37. [Crossref] [PubMed]
- Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007;1:313-23. [Crossref] [PubMed]
- Steele CW, Karim SA, Leach JD, et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell 2016;29:832-45. [Crossref] [PubMed]
- Nywening TM, Wang-Gillam A, Sanford DE, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol 2016;17:651-62. [Crossref] [PubMed]
- Wang-Gillam A, Noel MS, Sleijfer S, et al. The inhibition of CCR2 to modify the microenvironment in pancreatic cancer mouse model and to support the profiling of the CCR2 inhibitor CCX872-B in patients. J Clin Oncol 2016;34:suppl; abstr e15743.
- Noel MS, Hezel AF, Linehan D, et al. Orally administered CCR2 selective inhibitor CCX872-b clinical trial in pancreatic cancer. J Clin Oncol 2017;35:suppl 4S; abstract 276.
- Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 2014;74:5057-69. [Crossref] [PubMed]
- Ries CH, Cannarile MA, Hoves S, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 2014;25:846-59. [Crossref] [PubMed]
- Witkiewicz A, Williams TK, Cozzitorto J, et al. Expression of indoleamine 2,3-dioxygenase in metastatic pancreatic ductal adenocarcinoma recruits regulatory T cells to avoid immune detection. J Am Coll Surg 2008;206:849-54; discussion 54-6. [Crossref] [PubMed]
- Liu X, Shin N, Koblish HK, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 2010;115:3520-30. [Crossref] [PubMed]
- Gangadhar TC, Hamid O, Smith DC, et al. Epacadostat plus pembrolizumab in patients with advanced melanoma and select solid tumors: Updated phase 1 results from ECHO-202/KEYNOTE-037. Ann Oncol 2016;27:1110PD.
- Bahary N, Garrido-Laguna I, Cinar P, et al. Phase 2 trial of the indoleamine 2, 3-dioxygenase pathway (IDO) inhibitor indoximod plus gemcitabine/nab-paclitaxel for the treatment of metastatic pancreas cancer: interim analysis. J Clin Oncol 2016;34:suppl; abstr 3020.
- Hsu YC, Li L, Fuchs E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 2014;157:935-49. [Crossref] [PubMed]
- Bailey JM, Mohr AM, Hollingsworth MA. Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene 2009;28:3513-25. [Crossref] [PubMed]
- Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457-61. [Crossref] [PubMed]
- Lee JJ, Perera RM, Wang H, et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci U S A 2014;111:E3091-100. [Crossref] [PubMed]
- Macarulla T, Tabernero J, Palmer DH, et al. A phase Ib dose escalation, safety, and tolerability study of sonidegib in combination with gemcitabine in patients with locally advanced or metastatic pancreatic adenocarcinoma. J Clin Oncol 2016;34 suppl:371. [Crossref]
- Catenacci DV, Junttila MR, Karrison T, et al. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer. J Clin Oncol 2015;33:4284-92. [Crossref] [PubMed]
- Infinity Reports Update from Phase 2 Study of Saridegib Plus Gemcitabine in Patients with Metastatic Pancreatic Cancer. Available online: http://www.businesswire.com/news/home/20120127005146/en/Infinity-Reports-Update-Phase-2-Study-Saridegib
- De Jesus-Acosta A, O'Dwyer PJ, Ramanathan RK, et al. A phase II study of vismodegib, a hedgehog (Hh) pathway inhibitor, combined with gemcitabine and nab-paclitaxel (nab-P) in patients (pts) with untreated metastatic pancreatic ductal adenocarcinoma (PDA). J Clin Oncol 2014;32:suppl 3; abstr 257.
- Nielsen MF, Mortensen MB, Detlefsen S. Key players in pancreatic cancer-stroma interaction: Cancer-associated fibroblasts, endothelial and inflammatory cells. World J Gastroenterol 2016;22:2678-700. [Crossref] [PubMed]
- Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014;159:80-93. [Crossref] [PubMed]
- Albeituni SH, Ding C, Yan J. Hampering immune suppressors: therapeutic targeting of myeloid-derived suppressor cells in cancer. Cancer J 2013;19:490-501. [Crossref] [PubMed]
- Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 2014;14:598-610. [Crossref] [PubMed]
- Stokes JB, Adair SJ, Slack-Davis JK, et al. Inhibition of focal adhesion kinase by PF-562,271 inhibits the growth and metastasis of pancreatic cancer concomitant with altering the tumor microenvironment. Mol Cancer Ther 2011;10:2135-45. [Crossref] [PubMed]
- Lee CJ, Dosch J, Simeone DM. Pancreatic Cancer Stem Cells. J Clin Oncol 2008;26:2806-12. [Crossref] [PubMed]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005;5:275-84. [Crossref] [PubMed]
- Shah AN, Summy JM, Zhang J, et al. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol 2007;14:3629-37. [Crossref] [PubMed]
- Xu W, Wang Z, Zhang W, et al. Mutated K-ras activates CDK8 to stimulate the epithelial-to-mesenchymal transition in pancreatic cancer in part via the Wnt/beta-catenin signaling pathway. Cancer Lett 2015;356:613-27. [Crossref] [PubMed]
- Asanuma K, Moriai R, Yajima T, et al. Survivin as a radioresistance factor in pancreatic cancer. Jpn J Cancer Res 2000;91:1204-9. [Crossref] [PubMed]
- Zhang Q, Meng XK, Wang WX, et al. The Wnt/beta-catenin signaling pathway mechanism for pancreatic cancer chemoresistance in a three-dimensional cancer microenvironment. Am J Transl Res 2016;8:4490-8. [PubMed]
- Zhang Y, Morris JP, Yan W, et al. Canonical wnt signaling is required for pancreatic carcinogenesis. Cancer Res 2013;73:4909-22. [Crossref] [PubMed]
- Le PN, McDermott JD, Jimeno A. Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther 2015;146:1-11. [Crossref] [PubMed]
- Ko AH, Chiorean EG, Kwak EL, et al. Final results of a phase Ib dose-escalation study of PRI-724, a CBP/beta-catenin modulator, plus gemcitabine (GEM) in patients with advanced pancreatic adenocarcinoma (APC) as second-line therapy after FOLFIRINOX or FOLFOX. J Clin Oncol 2016;34:suppl; abstr e15721.
- Hu H, Zhou L, Awadallah A, et al. Significance of Notch1-signaling pathway in human pancreatic development and carcinogenesis. Appl Immunohistochem Mol Morphol 2013;21:242-7. [PubMed]
- Thomas MM, Zhang Y, Mathew E, et al. Epithelial Notch signaling is a limiting step for pancreatic carcinogenesis. BMC Cancer 2014;14:862. [Crossref] [PubMed]
- Miyamoto Y, Maitra A, Ghosh B, et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell 2003;3:565-76. [Crossref] [PubMed]
- Abel EV, Kim EJ, Wu J, et al. The Notch pathway is important in maintaining the cancer stem cell population in pancreatic cancer. PLoS One 2014;9:e91983. [Crossref] [PubMed]
- Bao B, Wang Z, Ali S, et al. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett 2011;307:26-36. [Crossref] [PubMed]
- Yen WC, Fischer MM, Axelrod F, et al. Targeting Notch signaling with a Notch2/Notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor-initiating cell frequency. Clin Cancer Res 2015;21:2084-95. [Crossref] [PubMed]
- O'Reilly EM, Sahai V, Bendell JC, et al. Results of a randomized phase II trial of an anti-notch 2/3, tarextumab (OMP-59R5, TRXT, anti-Notch2/3), in combination with nab-paclitaxel and gemcitabine (Nab-P+Gem) in patients (pts) with untreated metastatic pancreatic cancer (mPC). J Clin Oncol 2017;35:suppl 4S; abstract 279.
- De Jesus-Acosta A, Laheru D, Maitra A, et al. A phase II study of the gamma secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Invest New Drugs 2014;32:739-45. [Crossref] [PubMed]
- Cook N, Basu B, Smith DM, et al. A phase I trial of the ɣ-secretase inhibitor (GSI) MK-0752 in combination with gemcitabine in patients with pancreatic ductal adenocarcinoma (PDAC). J Clin Oncol 2014;32 suppl:4116.
- Hidalgo M, Cooray P, Carrato A, et al. A phase 1b study of the anti-cancer stem cell agent demcizumab (DEM) and gemcitabine (GEM)+/-nab-paclitaxel in patients with pancreatic cancer. J Clin Oncol 2016;34 suppl:341. [Crossref]
- Yu JH, Kim H. Role of janus kinase/signal transducers and activators of transcription in the pathogenesis of pancreatitis and pancreatic cancer. Gut Liver 2012;6:417-22. [Crossref] [PubMed]
- Toyonaga T, Nakano K, Nagano M, et al. Blockade of constitutively activated Janus kinase/signal transducer and activator of transcription-3 pathway inhibits growth of human pancreatic cancer. Cancer Lett 2003;201:107-16. [Crossref] [PubMed]
- Corcoran RB, Contino G, Deshpande V, et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res 2011;71:5020-9. [Crossref] [PubMed]
- Hurwitz HI, Uppal N, Wagner SA, et al. Randomized, Double-Blind, Phase II Study of Ruxolitinib or Placebo in Combination With Capecitabine in Patients With Metastatic Pancreatic Cancer for Whom Therapy With Gemcitabine Has Failed. J Clin Oncol 2015;33:4039-47. [Crossref] [PubMed]
- Hurwitz H, Cutsem EV, Bendell JC, et al. Two randomized, placebo-controlled phase 3 studies of ruxolitinib (Rux) + capecitabine (C) in patients (pts) with advanced/metastatic pancreatic cancer (mPC) after failure/intolerance of first-line chemotherapy: JANUS 1 (J1) and JANUS 2 (J2). J Clin Oncol 2017;35:suppl 4S; abstract 343.
- Li Y, Rogoff HA, Keates S, et al. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci U S A 2015;112:1839-44. [Crossref] [PubMed]
- Shahda S, El-Rayes BF, O'Neil BH, et al. A phase Ib study of cancer stem cell (CSC) pathway inhibitor BBI-608 in combination with gemcitabine and nab-paclitaxel (nab-PTX) in patients (pts) with metastatic pancreatic ductal adenocarcinoma (mPDAC). J Clin Oncol 2016;34:suppl 4S; abstr 284.
- Bekaii-Saab TS, Mikhail S, Langleben A, et al. A phase Ib/II study of BBI608 combined with weekly paclitaxel in advanced pancreatic cancer. J Clin Oncol 2016;34:suppl 4S; abstr 196.
- Bekaii-Saab TS, Starodub A, El-Rayes BF, et al. A phase Ib/II study of cancer stemness inhibitor napabucasin (BBI-608) in combination with gemcitabine (gem) and nab-paclitaxel (nabPTX) in metastatic pancreatic adenocarcinoma (mPDAC) patients (pts). J Clin Oncol 2017;35:suppl; abstr 4106.
- Baker NM, Der CJ. Cancer: Drug for an 'undruggable' protein. Nature 2013;497:577-8. [Crossref] [PubMed]
- Zorde Khvalevsky E, Gabai R, Rachmut IH, et al. Mutant KRAS is a druggable target for pancreatic cancer. Proc Natl Acad Sci U S A 2013;110:20723-8. [Crossref] [PubMed]
- Golan T, Khvalevsky EZ, Hubert A, et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget 2015;6:24560-70. [Crossref] [PubMed]
- Van Cutsem E, Hidalgo M, Bazin I, et al. Phase II randomized trial of MEK inhibitor pimasertib or placebo combined with gemcitabine in the first-line treatment of metastatic pancreatic cancer. J Clin Oncol 2015;33:suppl 3; abstr 344.
- Riess H, Van Laethem JL, Martens UM, et al. Phase II study of the MEK inhibitor refametinib (BAY 86-9766) in combination with gemcitabine in patients with unresectable, locally advanced, or metastatic pancreatic cancer: Biomarker results. ASCO Annual Meeting, 2014.
- Bodoky G, Timcheva C, Spigel DR, et al. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Invest New Drugs 2012;30:1216-23. [Crossref] [PubMed]
- Infante JR, Somer BG, Park JO, et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer 2014;50:2072-81. [Crossref] [PubMed]
- Turke AB, Song Y, Costa C, et al. MEK inhibition leads to PI3K/AKT activation by relieving a negative feedback on ERBB receptors. Cancer Res 2012;72:3228-37. [Crossref] [PubMed]
- Tolcher AW, Bendell JC, Papadopoulos KP, et al. A phase IB trial of the oral MEK inhibitor trametinib (GSK1120212) in combination with everolimus in patients with advanced solid tumors. Ann Oncol 2015;26:58-64. [Crossref] [PubMed]
- Chung V, McDonough S, Philip PA, et al. Effect of Selumetinib and MK-2206 vs Oxaliplatin and Fluorouracil in Patients With Metastatic Pancreatic Cancer After Prior Therapy: SWOG S1115 Study Randomized Clinical Trial. JAMA Oncol 2017;3:516-22. [Crossref] [PubMed]
- Ko AH, Tempero MA, Bekaii-Saab TB, et al. Dual MEK/EGFR inhibition for advanced, chemotherapy-refractory pancreatic cancer: A multicenter phase II trial of selumetinib (AZD6244; ARRY-142886) plus erlotinib. ASCO Annual Meeting, 2013.
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 2009;9:153-66. [Crossref] [PubMed]
- Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene 2001;20:1803-15. [Crossref] [PubMed]
- O'Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol 2016;13:417-30. [Crossref] [PubMed]
- Feldmann G, Mishra A, Bisht S, et al. Cyclin-dependent kinase inhibitor Dinaciclib (SCH727965) inhibits pancreatic cancer growth and progression in murine xenograft models. Cancer Biol Ther 2011;12:598-609. [Crossref] [PubMed]
- Franco J, Witkiewicz AK, Knudsen ES. CDK4/6 inhibitors have potent activity in combination with pathway selective therapeutic agents in models of pancreatic cancer. Oncotarget 2014;5:6512-25. [Crossref] [PubMed]
- Seoane J, Gomis RR. TGF-beta Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb Perspect Biol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Drabsch Y, ten Dijke P. TGF-beta signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev 2012;31:553-68. [Crossref] [PubMed]
- Moz S, Basso D, Bozzato D, et al. SMAD4 loss enables EGF, TGFbeta1 and S100A8/A9 induced activation of critical pathways to invasion in human pancreatic adenocarcinoma cells. Oncotarget 2016;7:69927-44. [PubMed]
- Oettle H, Seufferlein T, Luger T, et al. Final results of a phase I/II study in patients with pancreatic cancer, malignant melanoma, and colorectal carcinoma with trabedersen. ASCO Annual Meeting, 2012.
- Melisi D, Garcia-Carbonero R, Macarulla T, et al. editors. A phase II, double-blind study of galunisertib+ gemcitabine (GG) vs gemcitabine+ placebo (GP) in patients (pts) with unresectable pancreatic cancer (PC). 2016 ASCO Annual Meeting J Clin Oncol 2016;34:abstract.
- McDonald OG, Li X, Saunders T, et al. Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat Genet 2017;49:367-76. [Crossref] [PubMed]
- Lee HS, Park SB, Kim SA, et al. A novel HDAC inhibitor, CG200745, inhibits pancreatic cancer cell growth and overcomes gemcitabine resistance. Sci Rep 2017;7:41615. [Crossref] [PubMed]
- Schadendorf D, Hodi FS, Robert C, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015;33:1889-94. [Crossref] [PubMed]
- Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti–Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non–Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. [Crossref] [PubMed]
- Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5:177ra38. [Crossref] [PubMed]
- Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725-33. [Crossref] [PubMed]
- Kite Announces Positive Topline Primary Results of Axicabtagene Ciloleucel from First Pivotal CAR-T Trial in Patients with Aggressive Non-Hodgkin Lymphoma (press release), 02/28/2017. Available online: http://ir.kitepharma.com/releasedetail.cfm?ReleaseID=1014817
- Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009;206:3015-29. [Crossref] [PubMed]
- Johansson H, Andersson R, Bauden M, et al. Immune checkpoint therapy for pancreatic cancer. World J Gastroenterol 2016;22:9457-76. [Crossref] [PubMed]
- Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med 2016;375:1767-78. [Crossref] [PubMed]
- Loos M, Giese NA, Kleeff J, et al. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett 2008;268:98-109. [Crossref] [PubMed]
- Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010;33:828-33. [Crossref] [PubMed]
- Kalyan A, Kircher SM, Mohindra NA, et al. Ipilimumab and gemcitabine for advanced pancreas cancer: A phase Ib study J Clin Oncol 2016;34:suppl; abstr e15747.
- Aglietta M, Barone C, Sawyer MB, et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol 2014;25:1750-5. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Kunk PR, Bauer TW, Slingluff CL, et al. From bench to bedside a comprehensive review of pancreatic cancer immunotherapy. J Immunother Cancer 2016;4:14. [Crossref] [PubMed]
- Segal NH, Antonia SJ, Brahmer JR, et al. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. J Clin Oncol 2014;32:suppl; abstr 3002.
- FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication (press release), 5/23/2017. Available online: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm
- DT Le, JN Uram, H Wang, et al. PD-1 blockade in mismatch repair deficient non-colorectal gastrointestinal cancers. J Clin Oncol 2016;34:suppl 4S; abstr 195.
- Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 2013;36:382-9. [Crossref] [PubMed]
- Kalbasi A, June CH, Haas N, et al. Radiation and immunotherapy: a synergistic combination. The Journal of clinical investigation 2013;123:2756-63. [Crossref] [PubMed]
- Abate-Daga D, Rosenberg SA, Morgan RA. Pancreatic cancer: Hurdles in the engineering of CAR-based immunotherapies. Oncoimmunology 2014;3:e29194. [Crossref] [PubMed]
- Hassan R, Thomas A, Alewine C, et al. Mesothelin Immunotherapy for Cancer: Ready for Prime Time? J Clin Oncol 2016;34:4171-9. [Crossref] [PubMed]
- Golfier S, Kopitz C, Kahnert A, et al. Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther 2014;13:1537-48. [Crossref] [PubMed]
- Blumenschein GR, Hassan R, Moore KN, et al. Phase I study of anti-mesothelin antibody drug conjugate anetumab ravtansine (AR). J Clin Oncol 2016;34:suppl; abstr 2509.
- O’Reilly EM, Bauer TM, Infante J, et al. Abstract CT026: Phase I trial of HuMab-5B1 (MVT-5873), a novel monoclonal antibody targeting sLea, in patients with advanced pancreatic cancer and other CA19-9 positive malignancies. AACR Annual Meeting 2016, New Orleans, LA, USA.
- O'Reilly EM, Wang JS, Yu KH, et al. Single agent HuMab-5B1 (MVT-5873), a monoclonal antibody targeting sLea, in patients with pancreatic cancer and other CA19-9 positive malignancies. J Clin Oncol 2017;35:suppl; abstr 4110.
- Rizvi NA, Loo D, Baughman JE, et al. A phase 1 study of enoblituzumab in combination with pembrolizumab in patients with advanced B7-H3-expressing cancers. J Clin Oncol 2016;34:suppl; abstr TPS3104.
- Gómez VE, Giovannetti E, Peters GJ. Unraveling the complexity of autophagy: Potential therapeutic applications in Pancreatic Ductal Adenocarcinoma. Semin Cancer Biol 2015;35:11-9. [Crossref] [PubMed]
- Seo JW, Choi J, Lee SY, et al. Autophagy is required for PDAC glutamine metabolism. Sci Rep 2016;6:37594. [Crossref] [PubMed]
- Bowles TL, Kim R, Galante J, et al. Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. Int J Cancer 2008;123:1950-5. [Crossref] [PubMed]
- Yang S, Wang X, Contino G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev 2011;25:717-29. [Crossref] [PubMed]
- Zachar Z, Marecek J, Maturo C, et al. Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. J Mol Med (Berl) 2011;89:1137-48. [Crossref] [PubMed]
- Alistar AT, Desnoyer R, D'Agostino R. The mitochondrial metabolism inhibitor CPI-613 in combination with mFOLFIRINOX for pancreatic adenocarcinoma. Gastrointestinal Cancers Symposium Meeting, 2016.
- Lowery MA, Yu KH, Kelsen DP, et al. A Phase 1/1B trial of ADI-PEG 20 plus Nab-Paclitaxel and Gemcitabine in Patients with Advanced Pancreatic Adenocarcinoma. Cancer 2017. In Press.
- ERYTECH reports positive Phase 2b data for eryaspase for the treatment of metastatic pancreatic cancer (press release), 3/27/2017 . Available online: http://erytech.com/pdf/2017/170327_ERYTECH_PR_Pancreatic_Phase2_Topline_Results_EN_vdef.pdf
- Salo-Mullen EE, O'Reilly EM, Kelsen DP, et al. Identification of germline genetic mutations in patients with pancreatic cancer. Cancer 2015;121:4382-8. [Crossref] [PubMed]
- Teo MY, O'Reilly EM. Is it time to split strategies to treat homologous recombinant deficiency in pancreas cancer? J Gastrointest Oncol 2016;7:738-49. [Crossref] [PubMed]
- Holter S, Borgida A, Dodd A, et al. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients With Pancreatic Adenocarcinoma. J Clin Oncol 2015;33:3124-9. [Crossref] [PubMed]
- Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 2013;14:197-210. [Crossref]
- Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol 2008;26:3785-90. [Crossref] [PubMed]
- Antoniou AC, Casadei S, Heikkinen T, et al. Breast-cancer risk in families with mutations in PALB2. N Engl J Med 2014;371:497-506. [Crossref] [PubMed]
- Golan T, Kanji ZS, Epelbaum R, et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 2014;111:1132-8. [Crossref] [PubMed]
- Vyas O, Leung K, Ledbetter L, et al. Clinical outcomes in pancreatic adenocarcinoma associated with BRCA-2 mutation. Anticancer Drugs 2015;26:224-6. [Crossref] [PubMed]
- Lowery MA, Kelsen DP, Stadler ZK, et al. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist 2011;16:1397-402. [Crossref] [PubMed]
- Cavallo F, Graziani G, Antinozzi C, et al. Reduced proficiency in homologous recombination underlies the high sensitivity of embryonal carcinoma testicular germ cell tumors to Cisplatin and poly (adp-ribose) polymerase inhibition. PLoS One 2012;7:e51563. [Crossref] [PubMed]
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244-50. [Crossref] [PubMed]
- Rahib L, Fleshman JM, Matrisian LM, et al. Evaluation of Pancreatic Cancer Clinical Trials and Benchmarks for Clinically Meaningful Future Trials: A Systematic Review. JAMA Oncol 2016;2:1209-16. [Crossref] [PubMed]
- Jardim DL, Groves ES, Breitfeld PP, et al. Factors associated with failure of oncology drugs in late-stage clinical development: A systematic review. Cancer Treat Rev 2017;52:12-21. [Crossref] [PubMed]


