Best practices for the treatment of metastatic pancreatic adenocarcinoma: the therapeutic landscape in 2017
Introduction
Pancreatic cancer represents the seventh most common cause of cancer mortality worldwide, accounting for about 4% of cancer-related deaths in both males and females, with a global burden that is predicted to rise significantly over the next 15 years in all geographic regions of the world (1). In Western countries, the scope of the problem is even greater; based on current projections, pancreatic cancer is expected to surpass colorectal cancer within the next decade as the second leading cause of cancer mortality in the United States, behind only lung cancer (2).
More than half of patients with pancreatic cancer have metastatic disease at the time of presentation, with an additional third having inoperable tumors based on the locally advanced nature of their disease (3). For these patients, in whom curative surgery does not represent a viable therapeutic option, the mainstay of treatment consists of systemic therapy, which should be initiated in as expedient fashion as safely possible given the often aggressive tempo of disease. By contrast, locoregional approaches, such as surgery and radiation, should generally not be used in the setting of metastatic pancreatic cancer, except in rare circumstances (such as palliative radiation to a site of bone metastasis causing intractable pain) when less invasive approaches prove ineffective. While there have been case series describing favorable outcomes for patients undergoing metastasectomy (primarily those with limited pulmonary metastases) (4-6) and primary pancreatic tumor resection (7), it is unclear whether such strategies truly confer any survival benefit, and thus cannot be recommended as a general principle. Highly selected individuals with oligometastatic disease who have demonstrated durable disease control following systemic therapy should ideally seek consultation at a high-volume center providing specialized multidisciplinary care in pancreatic cancer prior to considering any sort of locoregional intervention, particularly metastasectomy or tumor debulking procedure.
It is important to recognize that treatment in the context of metastatic disease is rarely if ever administered with curative intent, with relative 5-year survival rates for patients at this stage remaining less than 3% (3). Thus, the goals of therapy should be communicated clearly to patients: namely, to try to achieve as deep and durable a remission as possible that may translate into prolonged survival, while palliating cancer-related symptoms and hopefully preserving or improving quality of life in the process. Given the pain, inanition, anorexia, and depression that are often associated with a diagnosis of pancreatic cancer, a comprehensive supportive care plan that accompanies administration of systemic therapy is absolutely essential. Elements of supportive care should include regular titration of analgesic medications and consideration of celiac plexus neurolysis in cases of unremitting pain; as well as expedited referrals to a pain management specialist, nutritionist, physical therapist, and psychologist or psychiatrist as appropriate. Furthermore, co-management of patients with a gastroenterologist and/or interventional radiologist may be necessary in cases where the tumor is impeding normal biliary drainage or producing gastric outlet obstruction.
Despite these daunting challenges, there are still some reasons for optimism. Systemic treatment options have been evolving over the past decade for advanced/metastatic pancreatic cancer, as several new chemotherapy regimens have shown positive results in large randomized phase III clinical trials. This offers patients both more choices for treatment and greater opportunity to be sequenced through two or more lines of therapy. In this review, we will focus on currently available therapeutic options for metastatic pancreatic cancer, starting with a historical perspective and then moving onto a discussion of best practices for both first-line treatment and for treatment following progression on initial therapy, including the factors that should be used to guide selection.
Historical perspective: the early years of gemcitabine
The modern era of pancreatic cancer chemotherapy can be considered to have started when gemcitabine, a nucleoside analogue, was first approved by the United States Food and Drug Administration (FDA) for the treatment of metastatic pancreatic cancer in 1996. Prior to that time, the therapeutic approach for this disease typically centered on a fluorouracil (5-FU)-based regimen which, when administered in combination with leucovorin, was observed to have a variable response rate of 0–9% and a median survival in the range of 10–24 weeks (8,9).
The basis of the approval of gemcitabine was a phase III clinical trial of relatively modest sample size (n=126), in which previously untreated patients with advanced pancreatic cancer were randomized to receive either weekly infusions of gemcitabine or bolus 5-FU (10). Patients on the gemcitabine arm showed statistically significant improvements in both median survival (5.65 months compared to 4.41 months, respectively; P=0.0025) and 1-year survival rate (18% vs. 2%), although objective responses on both arms were rare (5.4% vs. 0%). Of perhaps equal or greater importance, gemcitabine-treated patients more frequently demonstrated alleviation of cancer-related symptoms, which represented the primary study endpoint. This qualify of life endpoint was defined by clinical benefit response (CBR), a composite of measurements indicating improvements in pain (analgesic consumption and pain intensity), Karnofsky performance status (KPS), and weight lasting at least 4 consecutive weeks. CBR was observed in 23.8% patients receiving gemcitabine compared to 4.8% of those receiving 5-FU (P=0.0022).
Thereafter, gemcitabine monotherapy was adopted as the reference standard in most subsequent studies of advanced pancreatic cancer. This drug is typically given as a 30-min infusion in 4-week cycles (weekly for 3 weeks, followed by 1 week off), although in some studies, including the registrational trial discussed above, the first treatment cycle consists of weekly infusions for 7 consecutive weeks, followed by a 1-week break. Several early efforts sought to optimize the drug delivery of gemcitabine by using a “fixed-dose rate” (FDR) approach, in which gemcitabine is administered at a FDR of 10 mg/m2/min (for a 1,000 mg/m2 dose, 100 min instead of the standard 30 min) to achieve a steady-state plasma levels greater than 20 mmol/L (11). This pharmacokinetically-guided strategy is intended to maximize intracellular accumulation of gemcitabine triphosphate, the active form of the drug. However, a subsequent phase III study failed to show the superiority of gemcitabine administered via FDR infusion compared either to standard-infusion gemcitabine or to a gemcitabine/platinum combination (12), and as a result this general approach has fallen out of widespread use.
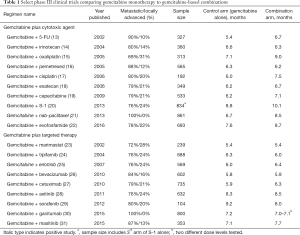
A large number of randomized phase III trials have been conducted evaluating the efficacy of various gemcitabine-based combinations in the first-line setting of advanced pancreatic cancer. Of note, many of the older studies included patients with both metastatic and locally advanced disease, an approach that has fallen out of favor as these stages are now being considered as separate and distinct entities for trial enrollment purposes. Strategies have included combining gemcitabine with both traditional cytotoxic agents, such as fluoropyrimidines, platinum analogues, camptothecins, taxanes, and alkylating agents; as well as with molecularly targeted agents intended to disrupt specific cancer cell signaling pathways governing survival, growth, metastatic spread, and angiogenesis (highlighted in Table 1). The vast majority of these trials failed to meet their primary endpoint of demonstrating an improvement in overall survival compared to gemcitabine alone.
Full table
Of the phase III studies reported through the first decade of the 2000s, the one positive trial worth noting is PA.3, a randomized clinical trial conducted by the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) (25). In this trial, 569 patients with locally advanced or metastatic pancreatic cancer were randomized to receive gemcitabine in combination with either erlotinib, an orally bioavailable epidermal growth factor receptor (EGFR) inhibitor, or placebo. A statistically significant survival advantage was observed for patients randomized to the erlotinib-containing arm [median survival 6.24 vs. 5.91 months; hazard ratio (HR) of 0.82, P=0.038], with the benefit most pronounced in those who developed grade 2 or higher rash (median survival 10.5 months). The positive results of this study led to FDA approval of erlotinib for the first-line treatment of metastatic pancreatic cancer (in combination with gemcitabine) in 2005, and it did represent an acceptable option in this setting. However, based on the small magnitude of improvement observed in the PA.3 study with what is perceived as a fairly marginal clinical benefit (as well as questionable value from a cost-effective perspective) (32), this gemcitabine/erlotinib combination is used somewhat infrequently at the present time, especially as it has been supplanted by other chemotherapy options producing better results in subsequent clinical trials, as discussed below.
Although most of the individual phase III trials of gemcitabine in combination with other cytotoxic agents failed to meet their primary endpoint, justification for use of doublet therapy has been provided by pooled analyses and meta-analyses of these studies. By amalgamating clinical trial data, a survival benefit can be observed when gemcitabine is combined with either a platinum analogue (cisplatin or oxaliplatin; HR 0.85, P=0.01) or a fluoropyrimidine (5-FU or capecitabine; HR 0.90, P=0.03) (33). The survival advantage of gemcitabine-fluorouracil combinations is even more pronounced when incorporating trials evaluating S-1, another oral fluoropyrimidine used primarily in Asian countries (HR 0.83, P<0.01) (34). Importantly, the benefit of combination therapy appears to be limited to patients with good performance status (HR 0.76, P<0.0001) (33), and not surprisingly, is associated with greater toxicities, particularly nausea, diarrhea, and cytopenias (35).
First-line treatment options in 2017
Substantial progress has been made in the clinical arena for the treatment of metastatic pancreatic cancer over the past several years. Specifically, positive results from two large phase III clinical trials have led to the establishment of two new combination chemotherapy regimens for patients with newly diagnosed metastatic disease: FOLFIRINOX and gemcitabine plus albumin-bound (nab)-paclitaxel.
FOLFIRINOX
The FOLFIRINOX regimen for pancreatic cancer (consisting of biweekly infusions of oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, irinotecan 180 mg/m2, and 5-FU administered both as a bolus at 400 mg/m2 and as an 46-h continuous infusion at 2,400 mg/m2) was originally developed by French investigators, based on preclinical and clinical evidence of antitumor activity of the individual components alone and in combination, as well as non-overlapping toxicities except for myelosuppression and diarrhea (36-38). A single-arm phase II trial (39) evaluating this regimen as first-line treatment enrolled 46 patients with locally advanced and metastatic pancreatic cancer across nine French centers, reporting an encouraging median survival of 10.2 months (9.5 months in the metastatic cohort). Moreover, the objective response rate (26%) was higher than that historically seen with gemcitabine, with a median response duration of 9.3 months and median progression-free survival of 8.2 months.
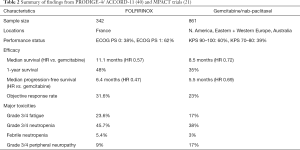
These promising results led to initiation of the phase III PRODIGE-4/ACCORD-11 trial (40), in which a total of 342 patients with previously untreated metastatic pancreatic cancer were randomized to receive either FOLFIRINOX or single-agent gemcitabine as first-line treatment. Importantly, study subjects were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 and to be no greater than 75 years of age, reflecting the fact that this new regimen under evaluation was (and is) intended for somewhat younger and fitter individuals. Additionally, fewer than 40% of patients enrolled on the trial had tumors located at the head of the pancreas, and as such, relatively few required endobiliary stenting. This distribution of pancreatic tumor location is the reverse of what is typically observed in clinical practice (41), and becomes relevant from a supportive care management standpoint when considering use of more aggressive chemotherapy. Specifically, one must exercise extra caution when selecting a more myelosuppressive regimen like FOLFIRINOX in patients with obstructing pancreatic head lesions and indwelling biliary stents, in whom the risks of ascending cholangitis and biliary sepsis are higher and stent management issues may need to be periodically addressed.
Nonetheless, PRODIGE-4/ACCORD-11 unequivocally showed the superiority of FOLFIRINOX over gemcitabine, with statistically significant improvements in median overall survival (11.1 vs. 6.8 months, HR for death, 0.57; P<0.001), progression-free survival (6.4 vs. 3.3 months, HR 0.47; P<0.001), and objective response rate (31.6% vs. 9.4%, P<0.001). An important quality of life instrument was embedded into the study design as well, indicating that while the side effect profile of FOLFIRINOX was not surprisingly more pronounced than gemcitabine (including a higher incidence of grade 3/4 neutropenia, febrile neutropenia, thrombocytopenia, diarrhea, and sensory neuropathy), it significantly reduced quality of life impairment and prolonged the time to definitive deterioration as measured by a number of functional and symptom domains (42).
As noted above, FOLFIRINOX should be limited to patients with good performance status. Due to concerns of significant toxicity associated with the original dosing and schedule, modified versions of FOLFIRINOX have also been developed that include omission of the 5-FU bolus component and empiric starting dose reduction of irinotecan by 25% (32,43,44). These modifications appear to produce comparable efficacy with improved tolerability compared to standard FOLFIRINOX, although formal comparative studies have not been performed. Furthermore, as irinotecan is extensively metabolized by the liver, relying primarily on biliary excretion, it may be necessary to start with FOLFOX alone for patients who present with obstructive jaundice and whose serum bilirubin levels remain elevated post-biliary stent placement, and to add irinotecan only if and once the bilirubin has sufficiently normalized. As a final practical measure, primary prophylaxis with pegfilgrastim should be considered as part of standard clinical practice for all patients receiving this regimen, as 42.5% of FOLFIRINOX-treated patients in the original phase III trial required growth factor support (40).
Gemcitabine plus nab-paclitaxel
An equally valid front-line option for patients with metastatic pancreatic cancer consists of the combination of gemcitabine and albumin-bound (nab)-paclitaxel. The phase I/II study examining this combination demonstrated substantial antitumor activity in patients with untreated metastatic pancreatic cancer; specifically, at the maximum tolerated dose (MTD) of gemcitabine 1,000 mg/m2 plus nab-paclitaxel 125 mg/m2 on a 3-week-on, 1-week-off schedule, the observed response rate was 48%, with a median overall survival of 12.2 months (n=44 patients treated at MTD) (45). Preclinical studies accompanying this original report showed that nab-paclitaxel appears to deplete the peritumoral desmoplastic stroma in patient-derived xenograft mouse models, possibly through targeting an albumin-binding protein called SPARC (secreted protein acidic and rich in cysteine), which then results in increased intratumoral concentration of gemcitabine.
These promising findings led to a large phase III trial called MPACT (Metastatic Pancreatic Adenocarcinoma Clinical Trial) (21). In this international study, conducted across 11 countries in North America, Europe, and Australia, a total of 861 patients with untreated metastatic pancreatic cancer and a KPS score between 70–100 were randomized in a 1:1 ratio to receive either gemcitabine plus nab-paclitaxel (at the MTD established in the phase I/II study) or gemcitabine alone.
The gemcitabine/nab-paclitaxel combination led to a significant improvement in median overall survival compared to gemcitabine monotherapy (8.5 vs. 6.7 months; HR for death 0.72, P<0.001). Other clinically relevant outcome measures, including median progression-free survival (5.5 vs. 3.7 months; HR 0.69, P<0.001), overall response rate (23% vs. 7%, P<0.001), and ≥90% decline in serum CA19-9 level (31% vs. 14%, P<0.001) all also favored the combination arm. Not surprisingly, the addition of nab-paclitaxel to gemcitabine led to higher rates of grade 3–4 neutropenia (38%), fatigue (17%), and peripheral neuropathy (17%), although the neuropathy was often reversible and nab-paclitaxel, when held, could later be resumed (at reduced doses) in 44% of subjects. Unlike the PRODIGE-4/ACCORD-11 trial, serial quality of life questionnaires were not performed as part of MPACT, although successor trials are specifically examining this quality of life issue in patients receiving gemcitabine/nab-paclitaxel (clinicaltrials.gov NCT02106884).
Criteria for choosing between FOLFIRINOX and gemcitabine/nab-paclitaxel
As FOLFIRINOX and gemcitabine/nab-paclitaxel have never been directly compared head-to-head in a prospective randomized study, these two chemotherapy regimens can essentially be considered co-equals as first-line treatment options for metastatic pancreatic cancer. A number of factors specific to the MPACT trial may help explain the slightly less impressive results conferred by gemcitabine/nab-paclitaxel when compared to FOLFIRINOX, including the broader geographic scope, larger sample size, wider age range (patients up to 88 years old were enrolled, with a median age of 63 years; compared to an upper age limit of 76 years with a median of 61 years in the PRODIGE study), and inclusion of poorer performance status patients (7.6% with a KPS of 60–70). However, the fact that the gemcitabine control arm for both studies produced the same median survival (6.7–6.8 months) suggests that the patient characteristics from both study cohorts were not markedly dissimilar. Acknowledging the pitfalls of cross-study comparisons, results from FOLFIRINOX and gemcitabine/nab-paclitaxel are shown side-by-side in Table 2.
At present, there is no predictive biomarker or molecular subgroup that allows one to choose between these two front-line regimens. As noted above, the albumin-bound protein SPARC has been hypothesized to mediate uptake of nab-paclitaxel and enhance drug delivery by depleting peritumoral stroma; in the phase I/II trial of gemcitabine plus nab-paclitaxel, higher levels of SPARC expression in stroma correlated with higher response rates and longer survival (45). However, the utility of SPARC as a predictive biomarker for nab-paclitaxel sensitivity was not validated in the pivotal phase III MPACT study, as it failed to show any significant association with response rate, progression-free survival, or overall survival in patients receiving combination therapy (46). There has also been considerable interest in hENT1 (human equilibrative nucleoside transporter 1) as a potential predictive marker for sensitivity to gemcitabine; however, in a large prospective randomized trial comparing a novel lipid-drug conjugate of gemcitabine (CO-101) to standard gemcitabine, no difference in survival was observed between the high and low hENT1 subgroups within the gemcitabine arm, failing to corroborate this as a useful predictor of gemcitabine outcome (47).
Meanwhile, no predictive markers have been confirmed to demonstrate sensitivity (or resistance) to FOLFIRINOX. However, pancreatic cancers associated with BRCA2 mutations or other genetic alterations indicative of homologous recombination deficiency may be uniquely sensitive to platinum agents (48,49). Therefore, for patients who are known mutant BRCA carriers, or who have a strong family history of cancer suggesting this possibility, it makes particular sense to start with FOLFIRINOX (or an alternative platinum-based combination, such as gemcitabine plus cisplatin). Of additional interest, preliminary clinical evidence further suggests that patients with BRCA-associated pancreatic cancer may potentially benefit from poly(ADP-ribose) polymerase (PARP) inhibitors such as olaparib (50), although this requires further validation in larger patient sets before it can be recommended for routine clinical practice.
Beyond this, providers must rely on clinical parameters in choosing the optimal therapy for any given patient, recognizing that it will be possible in some but not all patients to sequence them through both FOLFIRINOX and gemcitabine/nab-paclitaxel during the course of their treatment (discussed further below). There may be more latitude in using gemcitabine/nab-paclitaxel for older individuals and those with borderline performance status compared to FOLFIRINOX. Practical and logistic issues (such as the need for home infusions and port placement) may also mitigate against use of FOLFIRINOX in some patients. On the other hand, efficacy results from their respective phase III trials do appear to favor FOLFIRINOX slightly over gemcitabine/nab-paclitaxel, and on this basis many academic centers do lean toward starting with this regimen for patients with good performance status.
Value and cost considerations may also be taken into account in selecting between these two regimens, especially in resource-constrained areas of the world where access to either or both combinations may be limited by regulatory and/or reimbursement issues. Several cost effectiveness studies have been published exploring this question, taking into account not only chemotherapy costs but also other key factors such as hospitalizations for adverse events and supportive care costs (i.e., growth factors), with mixed results (51-53).
It is also important to recognize that a significant proportion of patients newly diagnosed with metastatic pancreatic cancer will not be candidates for either FOLFIRINOX or gemcitabine/nab-paclitaxel. One analysis from British Columbia, Canada examined a large, population-based, retrospective cohort of “real world” patients, and found that only 45% and 25% of individuals would have fulfilled eligibility requirements to receive treatment with FOLFIRINOX and gemcitabine/nab-paclitaxel, respectively, based on the specific eligibility criteria of the PRODIGE-4/ACCORD-11 and MPACT trials (54). The most common reasons for ineligibility included suboptimal performance status, elevated liver function tests, advanced age, and cardiac dysfunction. Treatment approaches for patients with poorer performance status, a very common scenario, are discussed further in the following section.
Options for patients with poorer performance status
As noted earlier, earlier meta-analyses have demonstrated that patients with poorer performance status (ECOG PS 2, KPS 60–80) are less likely to benefit from combination chemotherapy (11). A possible exception to this comes from subgroup analysis of the MPACT trial, in which patients with a KPS of 70–80 (translating roughly to an ECOG PS of 1–2), representing approximately 40% of the entire study population, still derived a survival benefit from combination therapy with gemcitabine/nab-paclitaxel (HR 0.61) (40). While this somewhat more aggressive approach can be tried in marginal patients with caution, there should be a low threshold to discontinue the nab-paclitaxel if excess toxicity develops. Patients with ECOG PS of 2 were also included in the PA.3 trial (representing 20% of the overall study cohort) (42); the HR of 0.61 for gemcitabine/erlotinib in this subgroup suggests that this combination could also be considered, additional toxicities notwithstanding.
For the majority of patients in this category, however, the most appropriate strategy is to treat with single-agent gemcitabine. This can be initiated at the standard 3-week-on, 1-week-off dosing schedule, but oftentimes it may necessary to switch to a 2-on/1-off or even an every other week schedule to provide patients more opportunity to recover between treatments. For those patients who may opt to avoid intravenous chemotherapy altogether, use of an oral fluoropyrimidine as a single agent may be a reasonable option. The strongest clinical data to support this approach centered on the oral fluoropyrimidine S-1, which combines tegafur, 5-chloro-2,4-dihydroxypyridine, and potassium oxonate, and is approved in many countries throughout Asia. In the 3-arm GEST trial (Gemcitabine and S-1 Trial) (20), 834 chemotherapy-naive patients with locally advanced or metastatic pancreatic cancer were randomized gemcitabine alone, S-1 alone, and the combination of gemcitabine and S-1. The non-inferiority of S-1 to gemcitabine was demonstrated (median overall survival of 9.7 vs. 8.8 months; HR 0.96, P<0.001 for non-inferiority), suggesting this may represent an alternative monotherapy option for patients in geographic areas where S-1 is available. In geographic areas where S-1 is not available, capecitabine (an oral prodrug of 5-FU) may represent a possible alternative, although phase III data are lacking to support this strategy. In one non-randomized phase II trial of 42 patients with untreated advanced or metastatic pancreatic cancer, capecitabine was associated with an objective response rate of 9.5% and a CBR of 24% (55).
Second-line therapy
Due to the clinical deterioration that often accompanies disease progression in metastatic pancreatic cancer, fewer than half of patients receiving first-line chemotherapy go on to receive any additional therapy. In the aforementioned PRODIGE-4/ACCORD-11 and MPACT trials, for example, only 48% and 40% of patients, respectively, received second-line treatment (39,45). For those individuals who remain well enough to consider further chemotherapy, selection of treatment is informed by a relatively limited number of randomized clinical trials in this setting and is dependent upon prior drug exposure, as patients may have received either a gemcitabine- or fluoropyrimidine-based regimen.
Most second-line trials have been conducted during the time and in the setting where gemcitabine (alone or in combination) has represented the standard first line of therapy. While a variety of agents have been investigated in the post-gemcitabine setting, including classical chemotherapy drugs, molecularly targeted therapies, and immuno-oncology agents, these have primarily been in the context of relatively small phase II clinical trials [reviewed in (56)]. The relatively small numbers of randomized phase III trials that have had the greatest impact on clinical practice have focused on fluoropyrimidine-based combinations.
5-FU plus oxaliplatin-based combinations
The combination of a fluoropyrimidine plus a platinum agent (primarily oxaliplatin) has represented the de facto standard of care over the past decade for patients with metastatic pancreatic cancer who have progressed on gemcitabine-based therapy. The most convincing evidence supporting this was a phase III randomized trial designed by Charité Onkologie (CONKO), a German cooperative group (43). In this CONKO-003 study, patients were randomized to receive either a regimen termed OFF (consisting of 24-h infusion 5-FU at 2,000 mg/m2 plus folinic acid 200 mg/m2 given weekly times 4 weeks, with the addition of oxaliplatin at 85 mg/m2 during weeks 2 and 4, in 6-week cycles); or weekly 5-FU/folinic acid (FF) alone. The results of this 168-patient trial were originally presented in abstract form in 2008 (57), but not published until 6 years later (58). Patients receiving OFF demonstrated a significant improvement in both median overall survival (5.9 vs. 3.3 months; HR 0.66, log-rank P=0.010) and progression-free survival (2.9 vs. 2.0 months; HR 0.68, log-rank P=0.019). Rates of neurotoxicity were higher in the oxaliplatin-containing arm, as expected, but otherwise the incidence of adverse events was similar between the two arms.
A separate randomized phase III Canadian trial (59) published subsequent to this reported results that were in almost direct contrast to CONKO-003. In this PANCREOX trial, a total of 108 patients with advanced pancreatic cancer (93% with metastatic disease) who had previously received gemcitabine-based chemotherapy were randomly assigned to receive either modified FOLFOX6 (bolus 5-FU 400 mg/m2, leucovorin 400 mg/m2, oxaliplatin 85 mg/m2, and 5-FU 2,400 mg/m2 as a 46-h continuous infusion); or biweekly infusional 5-FU/leucovorin. Somewhat surprisingly, no difference was observed between the two arms in terms of the primary study endpoint of median progression-free survival (3.1 vs. 2.9 months; HR 1.0, log-rank P=0.99); while median overall survival was actually worse in the mFOLFOX6 arm (6.1 vs. 9.9 months; HR 1.78, log-rank P=0.02). Possible explanations for why the addition of oxaliplatin might have been detrimental in this study include a higher incidence of grade 3/4 adverse events leading to withdrawal from treatment, more frequent dose delays and dose reductions, and a smaller proportion of patients going on to receive post-progression therapy.
While the OFF and mFOLFOX6 regimens differ from one another slightly, it is difficult to imagine that dosing/dose schedules alone could account for the very disparate results observed in the CONKO-003 and PANCREOX trials. Thus, while 5-FU plus oxaliplatin combinations continue to be used quite frequently in this second-line setting following gemcitabine-based chemotherapy, the actual magnitude of benefit (if any) remains very much in question. A small minority of patients may even remain fit enough to receive FOLFIRINOX at either full or attenuated doses, although this approach has only been looked at in a small number of retrospective case series rather than in prospective randomized fashion (60,61).
Nanoliposomal irinotecan (nal-IRI) plus 5-FU/leucovorin
nal-IRI was originally developed using an efficient high loading capacity system to encapsulate irinotecan within a nanoparticle/liposome carrier (62). This resulted in a drug with improved biodistribution and pharmacokinetic characteristics compared to free irinotecan, including prolonged exposure of SN-38 (the active metabolite of irinotecan) within tumors, as well as less systemic toxicity [reviewed in (63)].
The original pancreatic cancer-specific trial of nal-IRI consisted of a single-arm phase 2 study in patients with metastatic disease who had progressed on front-line gemcitabine-based chemotherapy (64). In this 40-patient trial, nal-IRI as a single agent produced an objective response rate of 7.5%, with median progression-free and overall survival of 2.4 and 5.2 months, respectively. This was felt to represent enough of a signal of clinical activity to warrant conduct of an international phase III trial (NAPOLI-1) (65) for this same patient population, in which 3 treatment arms were compared: (I) nal-IRI alone (120 mg/m2 on an every 3-week schedule); (II) a control arm of 24-h infusion 5-FU plus leucovorin (administered weekly for 4 out of 6 weeks); and (III) the combination of nal-IRI (80 mg/m2), 46-h infusion 5-FU (2,400 mg/m2), and leucovorin (400 mg/m2), administered in 2-week cycles.
In total, 417 patients were enrolled on NAPOLI-1 across 14 countries worldwide, all of whom had received prior gemcitabine-based chemotherapy (55% as combination therapy), and 34% who had received more than one prior line of treatment. Patients receiving the combination of nal-IRI and 5-FU/leucovorin showed a higher median overall survival compared to those receiving 5-FU/leucovorin alone (6.1 vs. 4.2 months; HR 0.67, P=0.012). Other clinically relevant outcome measures, including median progression-free survival (3.1 vs. 1.5 months; HR 0.56, P=0.0001), objective response rate (16% vs. 1%, P<0.0001), and CA19-9 decline of ≥50% from baseline (29% vs. 9%, P<0.0001) all also significantly favored the combination arm. Nal-IRI by itself, on the other hand, did not significantly improve survival compared to 5-FU/leucovorin (4.9 vs. 4.2 months; HR 0.99, P=0.94). In general, combination therapy was reasonably well-tolerated, with the most common grade 3/4 adverse events associated with this regimen including neutropenia (27%), fatigue (14%), diarrhea (13%), and vomiting (11%).
On the basis of these positive results, the FDA approved nal-IRI (in combination with 5-FU/leucovorin) in October 2015 specifically for patients with metastatic pancreatic cancer following progression on gemcitabine-based therapy. While this represented an important clinical development in that nal-IRI represents the first therapeutic agent to receive approval in this disease specifically for use in the second-line setting and beyond, several outstanding questions still remain about the appropriate use and breadth of applicability of this agent. For one, as gemcitabine/nab-paclitaxel is now used quite widely in metastatic pancreatic cancer (noting that few patients on the NAPOLI-1 trial received this as part of their original regimen), will the selection and evolution of front-line treatment be expected in any way to affect the reported efficacy and safety of nal-IRI in the salvage setting? Furthermore, can we extrapolate from the results of NAPOLI-1 to assume that FOLFIRI would produce similar results (relevant, for example, in countries where nal-IRI is not available); or are the superior pharmacokinetic properties of nal-IRI really a critical factor in the positive clinical findings? Indeed, FOLFIRI has been evaluated in several small prospective and retrospective studies in this second-line setting, with results that appear comparable to those reported in NAPOLI-1 (66-68). However, absent formal direct head-to-head testing of FOLFIRI vs. nal-IRI plus 5-FU/leucovorin in prospective randomized study design, we are left with uncertainty regarding the superiority (or non-inferiority) of one regimen compared to the other.
A small proportion of patients may even be suitable candidates for third-line treatment, although very few studies specific to this clinical scenario have been reported. Such individuals, many of whom are likely to have more indolent tumor biology, should be steered toward appropriate clinical trials whenever possible. As far as standard of care therapies go, a logical sequence might include gemcitabine plus nab-paclitaxel as front- line therapy; followed by nal-IRI plus 5-FU/leucovorin in the second-line; and then, if the patient remains fit enough and wishes to remain proactive with further treatment, FOLFOX as a third-line salvage regimen.
Treatment following front-line FOLFIRINOX
Conversely, for patients who have started on FOLFIRINOX as their first line of therapy, switching to a gemcitabine-based regimen at the point of progression represents the next logical step. It is unclear whether combining gemcitabine with a second agent (such as nab-paclitaxel, capecitabine, or a platinum agent) confers better results than gemcitabine alone, and this decision should be based on the patient’s performance status as well as on cumulative toxicities (particularly cytopenias and peripheral neuropathy) that occurred during front-line therapy. The combination of gemcitabine and nab-paclitaxel post-FOLFIRINOX has been reported in several small studies (69-71). In one prospective multicenter cohort from the French AGEO (Association des Gastro-Entérologues Oncologues) group, 57 patients were treated with gemcitabine/nab-paclitaxel; median overall survival was 8.8 months, median progression-free survival was 5.1 months, and objective response rate was 17.5%, indicating reasonable antitumor activity in this setting (71).
Conclusions and future directions
Patients with metastatic pancreatic cancer have more options available to them today than they did in the recent past, allowing a greater proportion to receive multiple lines of therapy and requiring more thoughtful decision-making on the part of the treating physician regarding how best to select the appropriate regimen to use. Unlike many other solid tumors, pancreatic cancer still lacks even a single validated predictive biomarker or a molecular classification system to help guide selection of therapy, although new classification schemes have been developed that may have practical application in the future in terms of both prognostic and, hopefully, therapeutic implications (72-74). Furthermore, clinical trials of novel agents offer promise that we will one day be able to move beyond standard chemotherapy drugs, including different classes of immuno-oncology (75-77), and stromal targeting agents (78). Ongoing trials that have reached phase III development are shown in Table 3. Building upon the recent modest successes we have achieved in the clinical management of this disease will require prioritizing clinical trial enrollment, whenever possible, over standard of care treatment.

Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Are C, Chowdhury S, Ahmad H, et al. Predictive global trends in the incidence and mortality of pancreatic cancer based on geographic location, socio-economic status, and demographic shift. J Surg Oncol 2016;114:736-42. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- SEER Cancer Stat Facts: Pancreas Cancer. National Cancer Institute. Bethesda M. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html
- Arnaoutakis GJ, Rangachari D, Laheru DA, et al. Pulmonary resection for isolated pancreatic adenocarcinoma metastasis: an analysis of outcomes and survival. J Gastrointest Surg 2011;15:1611-7. [Crossref] [PubMed]
- Tagawa T, Ito K, Fukuzawa K, et al. Surgical Resection for Pulmonary Metastasis from Pancreatic and Biliary Tract Cancer. Anticancer Res 2017;37:1413-6. [Crossref] [PubMed]
- Robinson LA, Tanvetyanon T, Springett G, et al. Pulmonary metastasectomy for suspected pancreaticobiliary cancer. J Thorac Cardiovasc Surg 2016;152:75-82. [Crossref] [PubMed]
- Wright GP, Poruk KE, Zenati MS, et al. Primary Tumor Resection Following Favorable Response to Systemic Chemotherapy in Stage IV Pancreatic Adenocarcinoma with Synchronous Metastases: a Bi-institutional Analysis. J Gastrointest Surg 2016;20:1830-5. [Crossref] [PubMed]
- DeCaprio JA, Mayer RJ, Gonin R, et al. Fluorouracil and high-dose leucovorin in previously untreated patients with advanced adenocarcinoma of the pancreas: results of a phase II trial. J Clin Oncol 1991;9:2128-33. [Crossref] [PubMed]
- Van Rijswijk RE, Jeziorski K, Wagener DJ, et al. Weekly high-dose 5-fluorouracil and folinic acid in metastatic pancreatic carcinoma: a phase II study of the EORTC GastroIntestinal Tract Cancer Cooperative Group. Eur J Cancer 2004;40:2077-81. [Crossref] [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [Crossref] [PubMed]
- Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol 2003;21:3402-8. [Crossref] [PubMed]
- Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2009;27:3778-85. [Crossref] [PubMed]
- Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 2002;20:3270-5. [Crossref] [PubMed]
- Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 2004;22:3776-83. [Crossref] [PubMed]
- Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 2005;23:3509-16. [Crossref] [PubMed]
- Oettle H, Richards D, Ramanathan RK, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol 2005;16:1639-45. [Crossref] [PubMed]
- Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 2006;24:3946-52. [Crossref] [PubMed]
- Abou-Alfa GK, Letourneau R, Harker G, et al. Randomized phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer. J Clin Oncol 2006;24:4441-7. [Crossref] [PubMed]
- Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 2009;27:5513-8. [Crossref] [PubMed]
- Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013;31:1640-8. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Van Cutsem E, Lenz HJ, Furuse J, et al. MAESTRO: A randomized, double-blind phase III study of evofosfamide (Evo) in combination with gemcitabine (Gem) in previously untreated patients (pts) with metastatic or locally advanced unresectable pancreatic ductal adenocarcinoma (PDAC). J Clin Oncol 2016;34 (suppl; abstr 4007).
- Bramhall SR, Schulz J, Nemunaitis J, et al. A doubleblind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer 2002;87:161-7. [Crossref] [PubMed]
- Van Cutsem E, van de Velde H, Karasek P, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol 2004;22:1430-8. [Crossref] [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [Crossref] [PubMed]
- Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2010;28:3617-22. [Crossref] [PubMed]
- Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol 2010;28:3605-10. [Crossref] [PubMed]
- Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol 2011;12:256-62. [Crossref] [PubMed]
- Gonçalves A, Gilabert M, Francois E, et al. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol 2012;23:2799-805. [Crossref] [PubMed]
- Fuchs CS, Azevedo S, Okusaka T, et al. A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial. Ann Oncol 2015;26:921-7. [Crossref] [PubMed]
- Deplanque G, Demarchi M, Hebbar M, et al. A randomized, placebo-controlled phase III trial of masitinib plus gemcitabine in the treatment of advanced pancreatic cancer. Ann Oncol 2015;26:1194-200. [Crossref] [PubMed]
- Mahaseth H, Brutcher E, Kauh J, et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 2013;42:1311-5. [Crossref] [PubMed]
- Heinemann V, Boeck S, Hinke A, et al. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 2008;8:82. [Crossref] [PubMed]
- Li Q, Yan H, Liu W, et al. Efficacy and safety of gemcitabine-fluorouracil combination therapy in the management of advanced pancreatic cancer: a meta-analysis of randomized controlled trials. PLoS One 2014;9:e104346. [Crossref] [PubMed]
- Ciliberto D, Botta C, Correale P, et al. Role of gemcitabine-based combination therapy in the management of advanced pancreatic cancer: a meta-analysis of randomised trials. Eur J Cancer 2013;49:593-603. [Crossref] [PubMed]
- Wagener DJ, Verdonk HE, Dirix LY, et al. Phase II trial of CPT-11 in patients with advanced pancreatic cancer, an EORTC early clinical trials group study. Ann Oncol 1995;6:129-32. [Crossref] [PubMed]
- Azrak RG, Cao S, Slocum HK, et al. Therapeutic synergy between irinotecan and 5-fluorouracil against human tumor xenografts. Clin Cancer Res 2004;10:1121-9. [Crossref] [PubMed]
- Ducreux M, Mitry E, Ould-Kaci M, et al. Randomized phase II study evaluating oxaliplatin alone, oxaliplatin combined with infusional 5-FU, and infusional 5-FU alone in advanced pancreatic carcinoma patients. Ann Oncol 2004;15:467-73. [Crossref] [PubMed]
- Conroy T, Paillot B, Francois E, et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer--a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol 2005;23:1228-36. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Artinyan A, Soriano PA, Prendergast C, et al. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford) 2008;10:371-6. [Crossref] [PubMed]
- Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol 2013;31:23-9. [Crossref] [PubMed]
- Lowery MA, Yu KH, Adel NG, et al. Activity of front-line FOLFIRINOX (FFX) in stage III/IV pancreatic adenocarcinoma (PC) at Memorial Sloan-Kettering Cancer Center (MSKCC). J Clin Oncol 2012;30 (suppl; abstr 4057).
- James ES, Yao X, Cong X, et al. Interim analysis of a phase II study of dose-modified FOLFIRINOX (mFOLFIRINOX) in locally advanced (LAPC) and metastatic pancreatic cancer (MPC). J Clin Oncol 2014;32 (suppl; abstr e15226).
- Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011;29:4548-54. [Crossref] [PubMed]
- Hidalgo M, Plaza C, Musteanu M, et al. SPARC Expression Did Not Predict Efficacy of nab-Paclitaxel plus Gemcitabine or Gemcitabine Alone for Metastatic Pancreatic Cancer in an Exploratory Analysis of the Phase III MPACT Trial. Clin Cancer Res 2015;21:4811-8. [Crossref] [PubMed]
- Poplin E, Wasan H, Rolfe L, et al. Randomized, multicenter, phase II study of CO-101 versus gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma: including a prospective evaluation of the role of hENT1 in gemcitabine or CO-101 sensitivity. J Clin Oncol 2013;31:4453-61. [Crossref] [PubMed]
- Golan T, Kanji ZS, Epelbaum R, et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 2014;111:1132-8. [Crossref] [PubMed]
- Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495-501. [Crossref] [PubMed]
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244-50. [Crossref] [PubMed]
- Cheng WH, Sadeghi S, Lenz HJ, et al. Comparative effectiveness of FOLFIRINOX (FOL) versus gemcitabine and nab-paclitaxel (GNP) for the first-line treatment of metastatic pancreatic cancer. J Clin Oncol 2016;34 (suppl 4S; abstr 306).
- Gharaibeh M, McBride A, Bootman JL, et al. Economic evaluation for the US of nab-paclitaxel plus gemcitabine versus FOLFIRINOX versus gemcitabine in the treatment of metastatic pancreas cancer. J Med Econ 2017;20:345-52. [Crossref] [PubMed]
- Zhou J, Zhao R, Wen F, et al. Cost-effectiveness analysis of treatments for metastatic pancreatic cancer based on PRODIGE and MPACT trials. Tumori 2016;2016:294-300.
- Peixoto RD, Ho M, Renouf DJ, et al. Eligibility of Metastatic Pancreatic Cancer Patients for First-Line Palliative Intent nab-Paclitaxel Plus Gemcitabine Versus FOLFIRINOX. Am J Clin Oncol 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Cartwright TH, Cohn A, Varkey JA, et al. Phase II study of oral capecitabine in patients with advanced or metastatic pancreatic cancer. J Clin Oncol 2002;20:160-4. [Crossref] [PubMed]
- Walker EJ, Ko AH. Beyond first-line chemotherapy for advanced pancreatic cancer: an expanding array of therapeutic options? World J Gastroenterol 2014;20:2224-36. [Crossref] [PubMed]
- Pelzer U, Kubica K, Stieler J, et al. A randomized trial in patients with gemcitabine refractory pancreatic cancer. Final results of the CONKO 003 study. J Clin Oncol 2008;26:4508. [Crossref]
- Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 2014;32:2423-9. [Crossref] [PubMed]
- Gill S, Ko YJ, Cripps C, et al. PANCREOX: A Randomized Phase III Study of 5-Fluorouracil/Leucovorin With or Without Oxaliplatin for Second-Line Advanced Pancreatic Cancer in Patients Who Have Received Gemcitabine-Based Chemotherapy. J Clin Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Assaf E, Verlinde-Carvalho M, Delbaldo C, et al. 5-fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncology 2011;80:301-6. [Crossref] [PubMed]
- Lee MG, Lee SH, Lee SJ, et al. 5-Fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with advanced pancreatic cancer who have progressed on gemcitabine-based therapy. Chemotherapy 2013;59:273-9. [Crossref] [PubMed]
- Drummond DC, Noble CO, Guo Z, et al. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res 2006;66:3271-7. [Crossref] [PubMed]
- Ko AH. Nanomedicine developments in the treatment of metastatic pancreatic cancer: focus on nanoliposomal irinotecan. Int J Nanomedicine 2016;11:1225-35. [Crossref] [PubMed]
- Ko AH, Tempero MA, Shan YS, et al. A multinational phase 2 study of nanoliposomal irinotecan sucrosofate (PEP02, MM-398) for patients with gemcitabine-refractory metastatic pancreatic cancer. Br J Cancer 2013;109:920-5. [Crossref] [PubMed]
- Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016;387:545-57. [Crossref] [PubMed]
- Yoo C, Hwang JY, Kim JE, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer 2009;101:1658-63. [Crossref] [PubMed]
- Gebbia V, Maiello E, Giuliani F, et al. Irinotecan plus bolus/infusional 5-Fluorouracil and leucovorin in patients with pretreated advanced pancreatic carcinoma: a multicenter experience of the Gruppo Oncologico Italia Meridionale. Am J Clin Oncol 2010;33:461-4. [Crossref] [PubMed]
- Zaniboni A, Aitini E, Barni S, et al. FOLFIRI as second-line chemotherapy for advanced pancreatic cancer: a GISCAD multicenter phase II study. Cancer Chemother Pharmacol 2012;69:1641-5. [Crossref] [PubMed]
- Zhang Y, Hochster H, Stein S, et al. Gemcitabine plus nab-paclitaxel for advanced pancreatic cancer after first-line FOLFIRINOX: single institution retrospective review of efficacy and toxicity. Exp Hematol Oncol 2015;4:29. [Crossref] [PubMed]
- Bertocchi P, Abeni C, Meriggi F, et al. Gemcitabine Plus Nab-Paclitaxel as Second-Line and Beyond Treatment for Metastatic Pancreatic Cancer: a Single Institution Retrospective Analysis. Rev Recent Clin Trials 2015;10:142-5. [Crossref] [PubMed]
- Portal A, Pernot S, Tougeron D, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer 2015;113:989-95. [Crossref] [PubMed]
- Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47-52. [Crossref] [PubMed]
- Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med 2011;17:500-3. [Crossref] [PubMed]
- Moffitt RA, Marayati R, Flate EL, et al. Virtual microdissection identifies distinct tumor- and stromaspecific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 2015;47:1168-78. [Crossref] [PubMed]
- Nywening TM, Wang-Gillam A, Sanford DE, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, nonrandomised, phase 1b trial. Lancet Oncol 2016;17:651-62. [Crossref] [PubMed]
- Beatty GL, Torigian DA, Chiorean EG, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res 2013;19:6286-95. [Crossref] [PubMed]
- Bahary N, Garridolaguna I, Cinar P, et al. Phase 2 trial of the indoleamine 2,3-dioxygenase pathway (IDO) inhibitor indoximod plus gemcitabine/nab-paclitaxel for the treatment of metastatic pancreas cancer: Interim analysis. J Clin Oncol 2016;34 (suppl 4S; abstr 3020).
- Bullock AJ, Hingorani SR, Wu XW, et al. Final analysis of stage 1 data from a randomized phase II study of PEGPH20 plus nab-Paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients (pts), utilizing Ventana companion diagnostic assay. J Clin Oncol 2016;34 (suppl; abstr 4104).