Multimodality management of borderline resectable pancreatic adenocarcinoma
Background
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal cancers. Despite developments in both detection and management of this disease over the past three decades, the 5-year overall survival (OS) rate of all patients diagnosed with it remains less than 8% (1). The poor prognosis of PDAC is mainly attributed to an inability to diagnose it at an early stage, a natural history characterized by relatively rapid disease progression and a responsiveness to current chemotherapeutic regimens that is generally poor (2,3). Surgical resection of the primary tumor and regional lymph nodes is often cited as the only treatment modality that is potentially curative, and surgery de novo followed by 6 months of systemic chemotherapy represents the standard of care for patients with tumors that appear to be technically resectable. However, the survival benefit associated with this strategy is severely compromised if cancer cells remain following resection, whether within the tumor bed or at distant systemic sites.
Borderline resectable pancreatic cancer (BRPC) represents a distinct clinical stage of PDAC. Patients with BRPC are at high-risk for a microscopically positive surgical resection and/or early treatment failure after an initial surgical approach due to a variety of tumor and/or patient related factors (4-6). Although the optimal sequence, duration and mode of preoperative therapy for this group of patients remains disputed due to the inherent limitations of previously published studies, consensus guidelines have recommended a multimodality approach to care that typically incorporates systemic chemotherapy followed by consolidative chemoradiation and radical resection (7-10).
Definition
The first definition of BRPC was an exclusively radiographic one. Tumors with an absence of a perivascular fat plane over 180° of the superior mesenteric artery (SMA) and/or superior mesenteric vein and/or portal vein (SMV/PV) persisting for a length of greater than 1 cm on cross-sectional imaging studies were considered “marginally resectable” (11). The concept of BRPC has since evolved. Despite differences in definitions that persist, the general focus has remained tumor anatomy—specifically the relationships between the primary tumor and the central mesenteric vasculature. At MD Anderson Cancer Center, however, we have established and use a comprehensive classification of “borderline resectable” disease that reflects derangements in the cancer’s anticipated behavior and the patient’s physiologic profile in addition to tumor anatomy (12). We categorize patients with BRPC into anatomic (type A), biologic (type B) and conditional (type C) variants.
BRPC type A, as we initially described, included patients with tumors characterized anatomically by one or more of the following: (I) tumor vessel interface (TVI) of ≤180° of the circumference of the SMA or celiac axis; (II) TVI of any degree of the circumference of a short segment of the hepatic artery, typically at the origin of the gastroduodenal artery; (III) short-segment occlusion of the SMV, PV or SMV-PV confluence that is amenable to vascular resection and reconstruction due to patent SMV and PV below and above the area of tumor-related occlusion (13) (Figure 1).
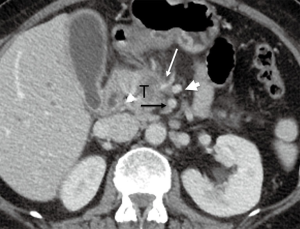
Although other anatomic definitions are currently used, there is agreement that some significant degree of reconstructable mesenteric vessel involvement by the tumor is the critical anatomic feature that positions BRPC between anatomically resectable and unresectable (locally advanced) tumors in the spectrum of localized disease (14). However, the anatomic definition utilized at MD Anderson differs slightly from the criteria proposed by investigators in the Alliance for Clinical Trials in Oncology, National Comprehensive Network, and the Americas Hepato-Pancreato-Biliary Association (AHPBA)/Society for Surgery of the Alimentary Tract (SSAT)/Society of Surgical Oncology (SSO) with respect to the degree to which apparent venous involvement discriminates between resectable and borderline resectable disease (10,12).
Patients with BRPC type B have clinical findings suspicious but not diagnostic for metastatic disease. These include indeterminate lesions on imaging in the liver or suspicious distant lymph nodes, serum carbohydrate antigen 19-9 (CA 19-9) level ≥1,000 U/mL in the setting of a normal bilirubin level or biopsy-proven involvement of regional lymph nodes (13).
BRPC type C patients require extensive assessment and optimization to undergo a major surgical procedure due to advance age (≥80 years old) or severe reversible pre-existing comorbidities or depressed performance status [Eastern Cooperative Oncology Group (ECOG) ≥2] (13,15).
A single patient may have features of one or more of these three variants.
Staging
At MD Anderson, all patients undergo an extensive history/physical examination and review of laboratory studies as part of a comprehensive evaluation at presentation to aid in identification of patients who are marginally resectable or inoperable based on anatomic or clinical criteria (16). Assessment of performance status is conducted using ECOG definitions (17) and comorbidities that may be a potential deterrent to a major abdominal surgery are identified (18,19).
Advances in cross-sectional imaging have led to a better assessment of TVI and disease extension and therefore resectability. The most commonly used modality is computed tomography (CT) with a standardized pancreatic protocol of the entire abdomen and pelvis to assess disease burden (20) with pre-contrast, late arterial and portal venous phases of enhancement that provide the ability to analyze the TVI (21). Enhanced magnetic resonance imaging (MRI) with gadolinium-based intravenous (IV) contrast administration is also effective in detecting local extension and TVI and is often superior to CT in detecting small liver lesions (22). However, due to higher cost, relative unavailability and expertise required for interpretation, MRI is typically used as a secondary modality in presence of liver lesion or CT contrast allergy or when CT cannot identify or characterize the pancreatic mass (4). Endoscopic ultrasound (EUS) is used to obtain tissue for diagnosis with fine-needle aspiration which is a pre-requisite for initiation of preoperative therapy and may actually be more sensitive for detection of small tumors, but we do not typically use it for staging purposes (23). Positron emission tomography (PET)-CT although not ideal for determining local tumor resectability, can be helpful to determine whether equivocal extra-pancreatic lesions truly represent metastases (24). During surgical exploration, occult metastatic disease has been reported in around 30% of patients with resectable disease on imaging, therefore staging laparoscopy is an important tool in preventing unnecessary pancreatectomy (25). It can be performed before initiation of therapy or in the preoperative setting either as a separate procedure or immediately before laparotomy under the same anesthetic (16,26). Selective use of staging laparoscopy has shown to be cost effective in high-risk patients (27). CA 19-9 level of ≥150 U/L and tumor size of ≥3 cm with radiographically localized disease has shown to be significant independent risk factors for unresectability (28,29). Since the peritoneum is one of the most frequent sites of failure in PDAC, it has been hypothesized that free cancer cells are present in the peritoneal cavity that later cause tumors to spread throughout the peritoneum (30), therefore in absence of other visible metastatic disease on imaging, examination of intraoperative peritoneal lavage cytology (PLC) may serve in identifying these. PLC has shown to be an independent prognostic factor for patients undergoing resection (31), with patients with positive PLC having similar survival as other patients with metastatic disease (32).
Response assessment
Serum CA 19-9 is the most commonly assayed tumor marker in clinical management of PDAC. Elevation of CA 19-9 is associated with poor survival, unresectability (33-35) and tumor stage (36). Previously, our group has shown that although the positive predictable value of a normal pre-treatment CA 19-9 value (<37 U/mL) for completion of neoadjuvant therapy and undergoing resection is close to 90%, its clinical utility is compromised by a low negative predictive value (33%) (37). Recently, we have shown CA 19-9 to be a dynamic marker of tumor biology and response to therapy, with strong association between a decrease of CA 19-9 following preoperative therapy and longer median OS among both unresected and resected patients (38). Additionally in the clinical setting, a ≥5% rise in CA 19-9 after 2 cycles of chemotherapy may serve as a negative predictive marker (39).
The 18F-fluorodeoxyglucose (FDG)-PET is a functional imaging method that is specific to metabolically active cancer cells which aid in predicting clinical outcomes based on baseline metabolic tumor activity and identifying response to treatment by assessing the viability of cancer cells following treatment (35,40-43).
Radiographic response and progression is routinely evaluated using Response Evaluation Criteria in Solid Tumor (RECIST) version 1.1 guidelines. These define complete response (CR) as the disappearance of visible tumor, partial response (PR) as at least a 30% reduction in tumor load and progressive disease (PD) as at least a 20% increase in tumor load or the appearance of a new lesion. Disease that does not meet the criteria for CR, PR or PD is defined as stable disease (SD) (44).
Radiographic downstaging has shown to be rare, although it may become more common as systemic chemotherapeutics and radiation regimens improve. Indeed, a prior study found only a 12% incidence of response meeting RECIST criteria after the administration of preoperative therapy (45). Furthermore, TVI may not change in a meaningful way after neoadjuvant therapy (46) and may persist even in patients with radiographic response (21). Patients with adequate functional status should be considered for surgery following the administration of preoperative therapy on the basis of lack of radiographic evidence for local or distant disease progression, even in the absence of downstaging or persistence of TVI (16).
Multimodality management
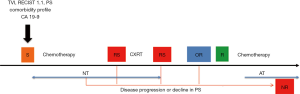
At MD Anderson, the general algorithm used in treatment of BRPC has historically been induction systemic chemotherapy with close monitoring for toxicity (47) followed by administration of chemoradiation (CXRT). Changes in either the radiographic findings or the patient’s clinical condition at the time of restaging necessitate a reassessment and revision of the treatment plan (14). In the absence of disease progression or decline in performance status at restaging after completion of CXRT, patients are taken to the operating room with intent of resection. Pancreatectomy is often followed by postoperative chemotherapy (48) (Figure 2). Patients are treated on clinical trials whenever possible.
Preoperative therapy
Reasonable rates of OS with use of preoperative therapy in patients with anatomically advanced PDAC when compared to patients receiving surgery first has been shown by multiple single institution retrospective review as well as an intention to treat analysis using the National Cancer Database (5,13,16,49-52). Selected studies highlighting current practices are illustrated in Table 1.

Full table
An animal study demonstrated that tagged pancreatic epithelial cells could be detected in the bloodstream and liver of mice with pre-invasive pancreatic lesions (57), suggesting that metastases may develop very early in the course of the disease—prior even to the growth of the primary tumor to a detectable size (14). This theory is further substantiated by the presence of radiographically occult metastatic disease found during laparotomy or laparoscopy in around 30% of patients (25) and high early recurrence rates even after margin-negative resections. In this context, perhaps the most important role of preoperative therapy is in providing a time interval in which to identify patients with suboptimal physiologic status and aggressive tumor biology, to avoid a pancreatectomy that is associated with little survival benefit.
A meta-analysis reported approximately one-third of patients who were deemed unresectable at initial staging may undergo neoadjuvant therapy and get “downstaged” to operable candidates while maintaining similar survival estimates as those initially deemed resectable (58). Additionally, reduction in the anatomical extent of tumor may help in facilitating a negative margin resection, which is widely accepted to strongly predict recurrence and survival (59-63). The presence of metastatic disease in peri-pancreatic lymph nodes has been shown to have an impact on survival (7,64). The lymph node ratio (LNR), defined as the number of lymph nodes with metastatic disease among the total number of lymph nodes retrieved, has been validated as a useful prognostic indicator and receiving neoadjuvant therapy has shown to be associated with reduced LNR in patients (65).
Chemotherapy
The rationale for use of systemic chemotherapy, commonly consisting of gemcitabine or 5-fluorouracil (5-FU) based regimens, includes early treatment of micrometastatic disease, possible downstaging and tumor response. Given the significant improvement in survival and response rates of 32% compared to 10% for gemcitabine alone with introduction of oxaliplatin and irinotecan to fluorouracil (FOLFIRINOX) in patients with metastatic disease (66), it has been a rational choice for induction therapy in patients with advanced non-metastatic disease and adequate performance status (50,53,54,67,68). A multicentric prospective study conducted by 20 centres analysing 47 patients with BRPC concluded the safety of resection after induction FOLFIRINOX with 30-day-mortality, major complications and symptomatic pancreatic fistula rates of 2.5%, 22.5% and 4% respectively, comparable to patients undergoing surgery de novo (69).
In addition to FOLFIRINOX, gemcitabine based regimens have also been shown to be effective in a preoperative setting (63). Mellon et al. reported resection and R0 rate of 51% and 96% respectively with estimated median OS of 19.2 months in 110 BRPC patients who received variable regimens, primarily gemcitabine in combination with docetaxel and capecitabine (GTX) followed by stereotactic body RT (SBRT) 40 Gy in 5 fractions (55). Addition of nab-paclitaxel to gemcitabine has shown promising results in metastatic patients (70) and prompted further exploration of the role of this combination in induction therapy, a recently concluded phase 1 study of 10 patients with BRPC reported resection rate of 80% and pathologic response of 30% comparable to previously published data with FOLFIRINOX. However the rate of ≥ grade 3 toxicity (the majority being neutropenia) was much higher at 90%. Additionally, radiographic response was not observed in any of the ten cases (56).
A phase II intergroup study Southwest Oncology Group (SWOG) Trial 1505 is currently recruiting participants and randomizing patients with resectable pancreatic cancer to three cycles of systemic FOLFIRINOX or three cycles of gemcitabine plus nab-paclitaxel. Patients without progression then undergo surgical resection followed by three cycles of the same regimen following surgery. The primary objective of this study is to pick the superior regimen with respect to OS (71). The results of this trial may inform future studies of borderline resectable disease.
Radiation therapy (RT)
The rationale for the use of RT in a preoperative setting is potential treatment of microscopic disease in regional lymph nodes and sterilization of the periphery of tumor to enhance the probability of negative margins at pancreatectomy (72-74). The standard approach at MD Anderson Cancer Center has historically been to use 50.4 Gy doses of RT in 28 fractions or 30 Gy in 10 fractions with conventional external beam RT (EBRT) and concurrent gemcitabine or 5-FU or capecitabine (75,76). In addition to the primary tumor, SMA and celiac axis should always be contoured and included within the margin (16). Recently, we have moved toward the use of SBRT, which is a modality designed to deliver high doses of RT precisely to small tumors, usually in five or fewer treatments. Intensity-modulated RT (IMRT) uses higher dose of radiation with the goal of varying intensities across the treatment field, and represents another option.
Retrospective single institution data suggests that SBRT is well tolerated and does not compromise potential surgery option or increase post-operative complications (55,77-79). Additionally, results of a single-institution phase 1 clinical trial with 13 patients suggests that SBRT after mFOLFIRINOX allows for higher radiation dose safely and may potentially aid in negative margin resections (80). Similarly, retrospective single institution studies suggest IMRT following induction chemotherapy may improve likelihood of R0 resection rate without compromising the organs at risk for toxicity (81,82). Though there is a theoretical advantage in using IMRT and SBRT, they have not proven to be more effective or to result in fewer side effects than standard RT (16).
Pancreatectomy and histopathologic assessment
Radical resection of the primary tumor and regional lymphadenectomy offers the only viable option for cure (83). Even following the administration of preoperative therapy, surgeons must anticipate the need for vascular resection and reconstruction during pancreatectomy for all patients with advanced cancers (21). Venous resection and reconstruction should be performed for borderline resectable tumors involving the SMV/PV as long as reasonable venous inflow and outflow is present and the surgeon feels that an R0 or R1 resection likely can be accomplished (84). When adequately planned for and performed by an experienced surgeon, vascular resection itself been shown to have no adverse impact on survival with postoperative morbidity, mortality rates and median survival of approximately 2 years, comparable with standard pancreatectomy procedures and superior to historical patients believed to have locally advanced disease treated palliatively (60,85,86). Nevertheless, arterial resection is associated with poor short and long-term outcome and may be justified but not recommended outside of clinical trials (7,87). Two primary surgical objectives in patients with borderline resectable PDAC include meticulous dissection along the peri-adventitial plane of the vessel to skeletonize SMA to maximize the potential for a margin-negative resection as cancer cells frequently infiltrate outward from the primary tumor toward the SMA through the perineural tissues in the retroperitoneum (76,88) and re-establishment of portal venous blood flow from the stomach and spleen, if necessary, to minimize the risk of postoperative sinistral portal hypertension (89). Since non-resectability is determined by involvement of the SMA, a need for early determination of resectability before an irreversible step, has promoted the development of an ‘artery-first’ approach (90) and although the operative technique and approach used vary, it has been shown to be safe and feasible in pancreatic resections and should be considered whenever tumor is thought to involve the SMV and/or PVs as a means to facilitate safe venous resection and reconstruction while preserving sound oncologic principles (91-93).
Margin status should be assessed intra-operatively by the surgeon and thoroughly evaluated by the pathologist. Each surgical specimen should be analyzed following standardized guidelines set by College of American Pathologist (CAP) guidelines and the American Joint Committee on Cancer (94-96). Specifically, the pancreatic and bile duct margins should be inked en face and are considered positive if tumor cells are present at the ink, whereas the entire inked SMA margin should be sectioned perpendicularly for microscopic evaluation and due to impact on outcome, the presence of tumor cells at or within 1 mm of the ink should also be considered a R1 resection (97). The treatment effect is measured histologically as the percentage of residual viable cancer cells and although varying staging scores are currently used, we use the modified CAP grading scheme as CR or minimal residual tumor (<5%) is an independent prognostic factor for patients receiving neoadjuvant therapy (98).
Postoperative therapy
Although survival benefits of adjuvant therapy after pancreatectomy in surgery de novo patients are widely accepted (99-101), the significance of postoperative therapy for patients who have already received preoperative therapy is unknown. Our group has previously shown that administration of postoperative therapy in these patients was associated with improved median OS (72 vs. 33 months; P=0.008) in absence of extensive metastatic disease in the regional lymph nodes (LNR <0.15). There was no association between postoperative chemotherapy and OS for patients with LNR ≥0.15, notably only 36 (14%) of the 263 patients identified received additional postoperative therapy (48).
Supportive therapy
In addition to a team of diagnostic radiologist, surgical oncologists, medical oncologists and radiation oncologists specializing in PDAC for accurate diagnosis and treatment; nutritionists, physical therapist and internist are integral in the management and optimization of functional status of BRPC patients.
Recent national studies
Currently available data consists of largely single-institutional retrospective reports that are limited by variability in patient cohort, definitions used, treatment regimens and absence of quality controls (12). Conducted by Alliance for Clinical Trials in Oncology, the recently concluded A021101 and currently enrolling A021501 have been designed with rigorous quality control of radiographic review, treatment modalities, the performance of surgery and histopathologic analysis providing a new standard for multi-institutional trials of preoperative therapy (9). In A021101, 22 patients with BRPC received neoadjuvant FOLFIRINOX followed by 5.5 weeks of EBRT, 50.4 Gy delivered in 28 daily fractions under A021101. Although 14 (64%) had a grade 3 or higher adverse event and only 27% showed RECIST response, 68% underwent pancreatectomy and negative resection margin was achieved in 93%. Pathologic response, defined as presence <5% viable tumor cells was reported in 33% of patients. Notably, one third of the resected patients did not start post-operative therapy further emphasising the importance of neoadjuvant therapy. The median OS of all patients from registration was 21.7 months (95% CI, 15.7 to not reached) and was comparable to previously reported OS of 21.2 months.
The currently enrolling A021501 trial is a randomized phase II study for BRPC of the head of the pancreas that compares two intensive preoperative therapy regimens. Patients receive either a systemic regimen of mFOLFIRINOX for eight cycles, or a combination regimen of seven cycles of mFOLFIRINOX followed by a 5-day radiation regimen using either SBRT or hypofractionated image-guided RT (102). The estimated enrollment is 134 patients and the purpose of this trial is to compare OS rates at 18 months, in addition to rate of pathologic response, toxicity and resection rate between a group receiving chemotherapy vs. chemotherapy and RT.
Conclusions
Although there is general consensus that preoperative therapy in patients with BRPC is beneficial, the optimal treatment regimen is unknown. Quality controlled prospective trials designed to overcome these limitations are paving the way for a more evidence-based management. The recently concluded intergroup study has shown the feasibility of such multicentric efforts as well as favorable resection rate and survival with use of multimodality therapy. Similar trials may aid in establishing an optimal strategy to achieve maximal outcomes in this high-risk group by further determining the most efficacious first line regimen and the roles of radiation and postoperative therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607-20. [Crossref] [PubMed]
- Tsai S, Christians KK, Ritch PS, et al. Multimodality Therapy in Patients With Borderline Resectable or Locally Advanced Pancreatic Cancer: Importance of Locoregional Therapies for a Systemic Disease. J Oncol Pract 2016;12:915-23. [Crossref] [PubMed]
- Schwarz L, Katz MH. Diagnosis and Management of Borderline Resectable Pancreatic Adenocarcinoma. Hematol Oncol Clin North Am 2015;29:727-40. [Crossref] [PubMed]
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035-46. [Crossref] [PubMed]
- Varadhachary GR, Abbruzzese JL. Novel approaches to 'borderline resectable' pancreatic tumors. Oncology (Williston Park) 2008;22:1529-30. [PubMed]
- Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2014;12:1083-93. [Crossref] [PubMed]
- Khorana AA, Mangu PB, Berlin J, et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:2541-56. [Crossref] [PubMed]
- Katz MH, Shi Q, Ahmad SA, et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg 2016;151:e161137. [Crossref] [PubMed]
- Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977-88. [Crossref] [PubMed]
- Mehta VK, Fisher G, Ford JA, et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J Gastrointest Surg 2001;5:27-35. [Crossref] [PubMed]
- Katz MH, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol 2013;20:2787-95. [Crossref] [PubMed]
- Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833-46; discussion 46-8. [Crossref] [PubMed]
- Cooper AB, Tzeng CW, Katz MH. Treatment of borderline resectable pancreatic cancer. Curr Treat Options Oncol 2013;14:293-310. [Crossref] [PubMed]
- Tzeng CW, Katz MH, Fleming JB, et al. Morbidity and mortality after pancreaticoduodenectomy in patients with borderline resectable type C clinical classification. J Gastrointest Surg 2014;18:146-55; discussion 55-6. [Crossref] [PubMed]
- Katz MH, Crane CH, Varadhachary G. Management of borderline resectable pancreatic cancer. Semin Radiat Oncol 2014;24:105-12. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Ragulin-Coyne E, Carroll JE, Smith JK, et al. Perioperative mortality after pancreatectomy: a risk score to aid decision-making. Surgery 2012;152:S120-7. [Crossref] [PubMed]
- Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004;291:2441-7. [Crossref] [PubMed]
- Karmazanovsky G, Fedorov V, Kubyshkin V, et al. Pancreatic head cancer: accuracy of CT in determination of resectability. Abdom Imaging 2005;30:488-500. [Crossref] [PubMed]
- Tran Cao HS, Balachandran A, Wang H, et al. Radiographic tumor-vein interface as a predictor of intraoperative, pathologic, and oncologic outcomes in resectable and borderline resectable pancreatic cancer. J Gastrointest Surg 2014;18:269-78; discussion 78. [Crossref] [PubMed]
- Balachandran A, Bhosale PR, Charnsangavej C, et al. Imaging of pancreatic neoplasms. Surg Oncol Clin N Am 2014;23:751-88. [Crossref] [PubMed]
- Dewitt J, Devereaux BM, Lehman GA, et al. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: a systematic review. Clin Gastroenterol Hepatol 2006;4:717-25. [Crossref] [PubMed]
- Delbeke D, Martin WH. PET and PET/CT for pancreatic malignancies. Surg Oncol Clin N Am 2010;19:235-54. [Crossref] [PubMed]
- Contreras CM, Stanelle EJ, Mansour J, et al. Staging laparoscopy enhances the detection of occult metastases in patients with pancreatic adenocarcinoma. J Surg Oncol 2009;100:663-9. [Crossref] [PubMed]
- Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727-33. [Crossref] [PubMed]
- Morris S, Gurusamy KS, Sheringham J, et al. Cost-effectiveness of diagnostic laparoscopy for assessing resectability in pancreatic and periampullary cancer. BMC Gastroenterol 2015;15:44. [Crossref] [PubMed]
- Satoi S, Yanagimoto H, Toyokawa H, et al. Selective use of staging laparoscopy based on carbohydrate antigen 19-9 level and tumor size in patients with radiographically defined potentially or borderline resectable pancreatic cancer. Pancreas 2011;40:426-32. [Crossref] [PubMed]
- De Rosa A, Cameron IC, Gomez D. Indications for staging laparoscopy in pancreatic cancer. HPB (Oxford) 2016;18:13-20. [Crossref] [PubMed]
- Konishi M, Kinoshita T, Nakagohri T, et al. Prognostic value of cytologic examination of peritoneal washings in pancreatic cancer. Arch Surg 2002;137:475-80. [Crossref] [PubMed]
- Hirabayashi K, Imoto A, Yamada M, et al. Positive Intraoperative Peritoneal Lavage Cytology is a Negative Prognostic Factor in Pancreatic Ductal Adenocarcinoma: A Retrospective Single-Center Study. Front Oncol 2015;5:182. [Crossref] [PubMed]
- Ferrone CR, Haas B, Tang L, et al. The influence of positive peritoneal cytology on survival in patients with pancreatic adenocarcinoma. J Gastrointest Surg 2006;10:1347-53. [Crossref] [PubMed]
- Ferrone CR, Finkelstein DM, Thayer SP, et al. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol 2006;24:2897-902. [Crossref] [PubMed]
- Nakao A, Oshima K, Nomoto S, et al. Clinical usefulness of CA-19-9 in pancreatic carcinoma. Semin Surg Oncol 1998;15:15-22. [Crossref] [PubMed]
- Barton JG, Bois JP, Sarr MG, et al. Predictive and prognostic value of CA 19-9 in resected pancreatic adenocarcinoma. J Gastrointest Surg 2009;13:2050-8. [Crossref] [PubMed]
- Bergquist JR, Puig CA, Shubert CR, et al. Carbohydrate Antigen 19-9 Elevation in Anatomically Resectable, Early Stage Pancreatic Cancer Is Independently Associated with Decreased Overall Survival and an Indication for Neoadjuvant Therapy: A National Cancer Database Study. J Am Coll Surg 2016;223:52-65. [Crossref] [PubMed]
- Katz MH, Varadhachary GR, Fleming JB, et al. Serum CA 19-9 as a marker of resectability and survival in patients with potentially resectable pancreatic cancer treated with neoadjuvant chemoradiation. Ann Surg Oncol 2010;17:1794-801. [Crossref] [PubMed]
- Tzeng CW, Balachandran A, Ahmad M, et al. Serum carbohydrate antigen 19-9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB (Oxford) 2014;16:430-8. [Crossref] [PubMed]
- Bauer TM, El-Rayes BF, Li X, et al. Carbohydrate antigen 19-9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: a pooled analysis of 6 prospective trials. Cancer 2013;119:285-92. [Crossref] [PubMed]
- Asagi A, Ohta K, Nasu J, et al. Utility of contrast-enhanced FDG-PET/CT in the clinical management of pancreatic cancer: impact on diagnosis, staging, evaluation of treatment response, and detection of recurrence. Pancreas 2013;42:11-9. [Crossref] [PubMed]
- Kauhanen SP, Komar G, Seppanen MP, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg 2009;250:957-63. [Crossref] [PubMed]
- Choi M, Heilbrun LK, Venkatramanamoorthy R, et al. Using 18F-fluorodeoxyglucose positron emission tomography to monitor clinical outcomes in patients treated with neoadjuvant chemo-radiotherapy for locally advanced pancreatic cancer. Am J Clin Oncol 2010;33:257-61. [PubMed]
- Bang S, Chung HW, Park SW, et al. The clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the differential diagnosis, staging, and response evaluation after concurrent chemoradiotherapy for pancreatic cancer. J Clin Gastroenterol 2006;40:923-9. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012;118:5749-56. [Crossref] [PubMed]
- Dholakia AS, Hacker-Prietz A, Wild AT, et al. Resection of borderline resectable pancreatic cancer after neoadjuvant chemoradiation does not depend on improved radiographic appearance of tumor-vessel relationships. J Radiat Oncol 2013;2:413-25. [Crossref] [PubMed]
- Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 2004;91:586-94. [Crossref] [PubMed]
- Roland CL, Katz MH, Tzeng CW, et al. The Addition of Postoperative Chemotherapy is Associated with Improved Survival in Patients with Pancreatic Cancer Treated with Preoperative Therapy. Ann Surg Oncol 2015;22 Suppl 3:S1221-8. [Crossref] [PubMed]
- Shubert CR, Bergquist JR, Groeschl RT, et al. Overall survival is increased among stage III pancreatic adenocarcinoma patients receiving neoadjuvant chemotherapy compared to surgery first and adjuvant chemotherapy: An intention to treat analysis of the National Cancer Database. Surgery 2016;160:1080-96. [Crossref] [PubMed]
- Hosein PJ, Macintyre J, Kawamura C, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer 2012;12:199. [Crossref] [PubMed]
- Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3496-502. [Crossref] [PubMed]
- Takahashi S, Kinoshita T, Konishi M, et al. Borderline resectable pancreatic cancer: rationale for multidisciplinary treatment. J Hepatobiliary Pancreat Sci 2011;18:567-74. [Crossref] [PubMed]
- Christians KK, Tsai S, Mahmoud A, et al. Neoadjuvant FOLFIRINOX for borderline resectable pancreas cancer: a new treatment paradigm? Oncologist 2014;19:266-74. [Crossref] [PubMed]
- Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol 2015;22:1153-9. [Crossref] [PubMed]
- Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol 2015;54:979-85. [Crossref] [PubMed]
- Okada KI, Hirono S, Kawai M, et al. Phase I Study of Nab-Paclitaxel plus Gemcitabine as Neoadjuvant Therapy for Borderline Resectable Pancreatic Cancer. Anticancer Res 2017;37:853-8. [Crossref] [PubMed]
- Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012;148:349-61. [Crossref] [PubMed]
- Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [Crossref] [PubMed]
- Chang DK, Johns AL, Merrett ND, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol 2009;27:2855-62. [Crossref] [PubMed]
- Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg 2004;8:935-49; discussion 49-50. [Crossref] [PubMed]
- Ethun CG, Kooby DA. The importance of surgical margins in pancreatic cancer. J Surg Oncol 2016;113:283-8. [Crossref] [PubMed]
- Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg 2007;246:52-60. [Crossref] [PubMed]
- Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3487-95. [Crossref] [PubMed]
- Fischer LK, Katz MH, Lee SM, et al. The number and ratio of positive lymph nodes affect pancreatic cancer patient survival after neoadjuvant therapy and pancreaticoduodenectomy. Histopathology 2016;68:210-20. [Crossref] [PubMed]
- Roland CL, Yang AD, Katz MH, et al. Neoadjuvant therapy is associated with a reduced lymph node ratio in patients with potentially resectable pancreatic cancer. Ann Surg Oncol 2015;22:1168-75. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Nanda RH, El-Rayes B, Maithel SK, et al. Neoadjuvant modified FOLFIRINOX and chemoradiation therapy for locally advanced pancreatic cancer improves resectability. J Surg Oncol 2015;111:1028-34. [Crossref] [PubMed]
- Boone BA, Steve J, Krasinskas AM, et al. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol 2013;108:236-41. [Crossref] [PubMed]
- Pietrasz D, Marthey L, Wagner M, et al. Pathologic Major Response After FOLFIRINOX is Prognostic for Patients Secondary Resected for Borderline or Locally Advanced Pancreatic Adenocarcinoma: An AGEO-FRENCH, Prospective, Multicentric Cohort. Ann Surg Oncol 2015;22 Suppl 3:S1196-205. [Crossref] [PubMed]
- Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011;29:4548-54. [Crossref] [PubMed]
- Sohal D. S1505: Combination Chemotherapy or Gemcitabine Hydrochloride and Paclitaxel Albumin-Stabilized Nanoparticle Formulation Before Surgery in Treating Patients With Pancreatic Cancer That Can Be Removed by Surgery. Available online: https://clinicaltrials.gov/show/NCT02562716
- Sasson AR, Wetherington RW, Hoffman JP, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: analysis of histopathology and outcome. Int J Gastrointest Cancer 2003;34:121-8. [Crossref] [PubMed]
- Stokes JB, Nolan NJ, Stelow EB, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol 2011;18:619-27. [Crossref] [PubMed]
- Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol 2001;8:123-32. [Crossref] [PubMed]
- Cloyd JM, Katz MH, Prakash L, et al. Preoperative Therapy and Pancreatoduodenectomy for Pancreatic Ductal Adenocarcinoma: a 25-Year Single-Institution Experience. J Gastrointest Surg 2017;21:164-74. [Crossref] [PubMed]
- Katz MH, Wang H, Balachandran A, et al. Effect of neoadjuvant chemoradiation and surgical technique on recurrence of localized pancreatic cancer. J Gastrointest Surg 2012;16:68-78; discussion 78-9. [Crossref] [PubMed]
- Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys 2013;86:516-22. [Crossref] [PubMed]
- Moningi S, Dholakia AS, Raman SP, et al. The Role of Stereotactic Body Radiation Therapy for Pancreatic Cancer: A Single-Institution Experience. Ann Surg Oncol 2015;22:2352-8. [Crossref] [PubMed]
- Herman JM, Koong AC. Stereotactic body radiation therapy: a new standard option for pancreatic cancer? J Natl Compr Canc Netw 2014;12:1489-93. [Crossref] [PubMed]
- Shaib WL, Hawk N, Cassidy RJ, et al. A Phase 1 Study of Stereotactic Body Radiation Therapy Dose Escalation for Borderline Resectable Pancreatic Cancer After Modified FOLFIRINOX (NCT01446458). Int J Radiat Oncol Biol Phys 2016;96:296-303. [Crossref] [PubMed]
- Huang X, Knoble JL, Zeng M, et al. Neoadjuvant Gemcitabine Chemotherapy followed by Concurrent IMRT Simultaneous Boost Achieves High R0 Resection in Borderline Resectable Pancreatic Cancer Patients. PLoS One 2016;11:e0166606. [Crossref] [PubMed]
- Badiyan SN, Olsen JR, Lee AY, et al. Induction Chemotherapy Followed by Concurrent Full-dose Gemcitabine and Intensity-modulated Radiation Therapy for Borderline Resectable and Locally Advanced Pancreatic Adenocarcinoma. Am J Clin Oncol 2016;39:1-7. [Crossref] [PubMed]
- Yamada S, Fujii T, Sugimoto H, et al. Aggressive surgery for borderline resectable pancreatic cancer: evaluation of National Comprehensive Cancer Network guidelines. Pancreas 2013;42:1004-10. [Crossref] [PubMed]
- Evans DB, Farnell MB, Lillemoe KD, et al. Surgical treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol 2009;16:1736-44. [Crossref] [PubMed]
- Riediger H, Makowiec F, Fischer E, et al. Postoperative morbidity and long-term survival after pancreaticoduodenectomy with superior mesenterico-portal vein resection. J Gastrointest Surg 2006;10:1106-15. [Crossref] [PubMed]
- Yekebas EF, Bogoevski D, Cataldegirmen G, et al. En bloc vascular resection for locally advanced pancreatic malignancies infiltrating major blood vessels: perioperative outcome and long-term survival in 136 patients. Ann Surg 2008;247:300-9. [Crossref] [PubMed]
- Mollberg N, Rahbari NN, Koch M, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg 2011;254:882-93. [Crossref] [PubMed]
- Butler JR, Ahmad SA, Katz MH, et al. A systematic review of the role of periadventitial dissection of the superior mesenteric artery in affecting margin status after pancreatoduodenectomy for pancreatic adenocarcinoma. HPB (Oxford) 2016;18:305-11. [Crossref] [PubMed]
- Katz MH, Lee JE, Pisters PW, et al. Retroperitoneal dissection in patients with borderline resectable pancreatic cancer: operative principles and techniques. J Am Coll Surg 2012;215:e11-8. [Crossref] [PubMed]
- Sanjay P, Takaori K, Govil S, et al. 'Artery-first' approaches to pancreatoduodenectomy. Br J Surg 2012;99:1027-35. [Crossref] [PubMed]
- Rose JB, Rocha F, Alseidi A, et al. Posterior 'superior mesenteric artery first' approach for resection of locally advanced pancreatic cancer. Ann Surg Oncol 2014;21:1927-8. [Crossref] [PubMed]
- Pessaux P, Varma D, Arnaud JP. Pancreaticoduodenectomy: superior mesenteric artery first approach. J Gastrointest Surg 2006;10:607-11. [Crossref] [PubMed]
- Zhu J, Han D, Li X, et al. Inferior Infracolic 'Superior Mesenteric Artery First' Approach with a No-Touch Isolation Surgical Technique in Patients with a Borderline Resectable Cancer of the Pancreatic Head. Ann Surg Oncol 2016;23:976-80. [Crossref] [PubMed]
- Protocol for the Examination of Specimens From Patients With Carcinoma of the Pancreas. College of American Pathologists. Available online: http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution Folders/WebContent/pdf/cp-pancreasexo-16protocol-3300.pdf
- Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg 2017;265:185-91.
- Katz MH, Merchant NB, Brower S, et al. Standardization of surgical and pathologic variables is needed in multicenter trials of adjuvant therapy for pancreatic cancer: results from the ACOSOG Z5031 trial. Ann Surg Oncol 2011;18:337-44. [Crossref] [PubMed]
- Liu L, Katz MH, Lee SM, et al. Superior Mesenteric Artery Margin of Posttherapy Pancreaticoduodenectomy and Prognosis in Patients With Pancreatic Ductal Adenocarcinoma. Am J Surg Pathol 2015;39:1395-403. [Crossref] [PubMed]
- Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer 2012;118:3182-90. [Crossref] [PubMed]
- Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol 2008;26:3503-10. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073-81. [Crossref] [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [Crossref] [PubMed]
- Alliance for Clinical Trials in Oncology. Preoperative Extended Chemotherapy vs. Chemotherapy Plus Hypofractionated Radiation Therapy for Borderline Resectable Adenocarcinoma of the Head of the Pancreas. Available online: https://clinicaltrials.gov/ct2/show/NCT02839343


