Epidemiology of pancreatic adenocarcinoma
Incidence and mortality
Pancreatic ductal adenocarcinoma (PDAC) is the 12th most common cancer worldwide with an age-standardized incidence rate of 4.2 per 100,000 and approximately 338,000 new cases diagnosed in 2012. Risk is higher in more developed regions than in those that are less developed, with age-standardized incidence rates of 7.2 and 2.8, respectively, per 100,000; about 55% of all pancreatic cancer cases are diagnosed in more developed regions. The highest incidence rates are found in Northern America (7.4 per 100,000) and Europe (6.8 per 100,000), with lower rates in Asia (3.2 per 100,000) and Africa (2 per 100,000) (1). Data from the United States SEER program (Surveillance, Epidemiology, and End Results) suggest that the incidence of pancreatic cancer has increased slightly over time (SEER).
Incidence is higher in men than in women, with global incidence rates per 100,000 of 4.9 for men and 3.6 for women, consistent across all regions. In 2012, approximately 178,000 men were diagnosed compared to 160,000 women. Older age is a strong risk factor for PDAC: the age-specific incidence rates of pancreatic cancer rise from 10.4 per 100,000 in those aged 55–59, to 24.0 in those aged 65–69, and to 55.7 in those aged ≥75 (1).
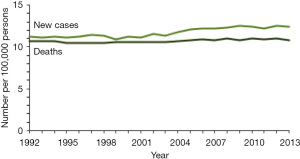
Worldwide, mortality rates for pancreatic cancer remain remarkably close to the incidence rates across geographic regions (1). Figure 1 illustrates the incidence of and mortality from PDAC in the US between 1995 and 2013, showing relatively stable patterns and close association between incidence and mortality. Globally, pancreatic cancer is the seventh leading cause of cancer deaths in both men and women, causing more than 330,000 deaths per year (2). High mortality rates are attributed to patients’ presenting with late stage disease at diagnosis. Based on SEER data, 52% of those diagnosed with pancreatic cancer present with Stage 4 disease, cancer that has already metastasized to other regions of the body; only 10% of those diagnosed present with localized disease (2,3). The SEER database reports the 5-year survival at around 7.7%, which has improved only slightly in recent years, in spite of newer treatments (2).
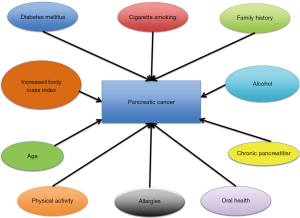
Risk factors for pancreatic cancer (PDAC)
Genetic and environmental factors are implicated in the development of PDAC. The main environmental factors associated with pancreatic cancer are shown in Figure 2. For this review, we informally searched the literature for recent reports on each of these risk factors. When possible, we focused our review on large pooled or meta-analyses encompassing several individual studies in order to provide more stable estimates of risk associations. When reviews or meta-analyses were not available, we relied on larger, more recent publications.
Cigarette smoking
There is a strong and consistent association between tobacco smoking and development of PDAC; prevention of PDAC is another reason to encourage individuals not to smoke. Tobacco smoke is a complex mixture of thousands of compounds, containing at least 50 known carcinogens, which likely initiate and facilitate the development of pancreatic tumors (4). A pooled analysis of 12 studies from the U.S and Europe, including >6,000 cases and >12,000 controls from the Pancreatic Cancer Case-Control Consortium (PanC4), examined the relationship between cigarette smoking and PDAC. Compared to those who had never smoked, current smokers had doubled risk [odds ratio (OR) =2.20, 95% confidence interval (CI) 1.71–2.83]; those who ever smoked had a 40% increased risk (OR =1.40, 95% CI 1.24–1.59); and former smokers had 17% higher risk (OR =1.17, 95% CI 1.02–1.34) (5). In a pooled analysis of cohort studies analyzed as nested case-control studies (with 1,481 cases and 1,539 controls) in the Pancreatic Cancer Cohort Consortium (PanScan), mostly from the US (6), Lynch and colleagues reported an OR =1.77 (95% CI 1.38–2.26) for current smokers and OR =1.24 (95% CI 1.06–1.46) for those who ever smoked, compared to those who never smoked. The stronger associations in the case-control studies reflect the design differences, with the case-control studies obtaining information on risk factors at the time of cancer diagnosis, and the cohort studies obtaining it at earlier times, not capturing smoking cessation between exposure assessment and cancer diagnosis.
In both of the studies (5,6), risk increased with increasing intensity (number of cigarettes per day), duration, and pack-years. In the PanC4 studies, risk for those who smoked ≥35 cigarettes per day was more than 3 times that of never smokers, and those who smoked ≥30 years were at more than double the risk. In the cohort studies, risk was highest in those with ≥30 cigarettes per day, >50 years of smoking, and >40 pack years. In contrast, a meta-analysis of 42 observational studies (7), focused primarily on intensity and duration of smoking, found that risk increased with the number of cigarettes up to 20 cigarettes per day, but did not increase substantially with higher numbers of cigarettes: relative risks (RR), compared to those who never smoked, were 1.5 (95% CI 1.4–1.6) for 10 cigarettes per day; RR =1.9 (95% CI 1.8–2.0) for 20 cigarettes per day; RR =2.0 (95% CI 1.9–2.1) for 30 cigarettes per day; and RR =2.1 (95% CI 1.9–2.3) for 40 cigarettes per day. Similar trends were noted for duration of smoking and pack years, with risk increasing more slowly at higher exposures. This meta-analysis included 30 case-control studies and 12 prospective studies from a wide range of locations, including several from Asia, with little overlap with the PanC4 and Lynch studies. The number of cases overall was >18,000, and the number of controls (including non-cases in cohort studies) was >2,100,000.
Risk of PDAC decreases with smoking cessation. The PanC4 pooled analysis found that 20 years after cessation, risk was equal to that of never smokers (5); the pooled cohort studies reported equal risk to never smokers after 15–20 years of quitting (6). The large meta-analysis (7) compared those who quit to current smokers, finding that those who quit experienced reduced risk as soon as the first year (RR =0.92, 95% CI 0.87–0.97), with this trend continuing, reaching RR =0.60 (95% CI 0.43–0.83) after 8 years. Overall, it is clear that smoking increases risk, and that quitting reverses this; differences in findings concerning increased risk with increased intensity and duration may reflect differences in the locations of the studies or other methodological factors.
High body mass index (BMI)
Another potentially modifiable factor implicated in the development of pancreas cancer is high BMI (weight in kg/height in meter2). Several studies have shown an association between being overweight or obese and risk of pancreas cancer; however, results are not always statistically significant in spite of large sample sizes. One mechanism hypothesized for high BMI leading to PDAC is that of insulin resistance (8).
In a meta-analysis of 23 cohort studies (9) including 9,504 cases, Aune and colleagues found that risk increased by 16% (95% CI 0.98–1.36) per 5 units of BMI. The association was not linear, with increased risk most evident at the highest levels of BMI, above 35. Another meta-analysis that included 20 cohort and case-control studies (10), with a total of greater than 6,000 PDAC cases, found that an increase of 5 kg/m2 in BMI was associated with increased risk in studies conducted in North America (RR =1.07, 95% CI 1.03–1.11) and Europe/Australia (RR =1.18, 95% CI 1.09–1.27), but not in studies in Asia or the Pacific (RR =0.94, 95% CI 0.71–1.24). In an earlier pooled analysis of 13 cohort studies in PanScan, with 2,170 cases and 2,209 controls, increased risk was reported for those classified as obese (BMI =30–34.9) and severely obese (BMI ≥35.0), with OR =1.28 (95% CI 0.99–1.67) and OR =1.53 (95% CI 0.99–2.36) respectively, with p for trend =0.003 (11). In another pooled analysis of data from cohort studies, being obese (BMI 30 to <40 kg/m2 compared with 21 to <23 kg/m2) in early adulthood (age 18–21) was associated with ~43% increase in pancreatic cancer mortality (HR =1.43, 95% CI 1.11–1.85), whereas weight gain later in life did not confer the same degree of risk (12).
Associations with central obesity have also been studied, with consistent and statistically significant results. In a pooled analysis of 20 cohort studies, those in the highest quartile of waist-to-hip ratio were at 28% higher risk of death from pancreatic cancer (95% CI 1.08–1.51) compared to those in the lowest quartile, after adjustment for BMI as well as other risk factors; the trend across quartiles was also statistically significant (12). In an earlier analysis, Aune et al. (9) found increased risk for a 0.1 unit increase in waist to hip ratio, with RR =1.19 (95% CI 1.09–1.31), and another earlier analysis reported RR =1.57 (95% CI 1.09–2.26) comparing the highest to lowest quartile of waist-to-hip ratio (11). While the latter two reports did not adjust for BMI, the one pooled analysis that did adjust (12) found only a small attenuation of the risk estimate. Overall, increased BMI, and particularly higher abdominal obesity, lead to additional risk of PDAC; reduction of the prevalence of overweight and obesity is a goal for prevention of pancreatic cancer as well as other diseases.
Family history, genetic mutations, and genetic polymorphisms
A family history of pancreatic cancer, usually defined as having at least one affected first degree relative, is seen in 5–10% of individuals with this disease (13). A pooled analysis of 10 cohort studies and one case-control study (14) found an OR =1.76 (95% CI 1.19–2.61) for those with a first degree relative with pancreatic cancer. A meta-analysis that included 7 case-control and 2 cohort studies (15) reported similar findings, with OR =1.80 (95% CI 1.48–2.12); there were stronger results in the case-control studies (RR =2.82, 95% CI 1.99–3.66) than in the cohort studies (RR =1.62, 95% CI 1.28–1.97). This may reflect greater awareness of family history among cases at the time of diagnosis of PDAC. In a registry-based study, risk was shown to increase strongly in families with several members affected (16).
Rare mutations in a number of genes are related to increased risk of PDAC. The most commonly found mutations in PDAC families are in BRCA2, with about twice the risk found in those with mutations in this gene (16). BRCA1 mutations are also associated with increased risk. Studies in cancer family registries indicate that families with carriers of BRCA2 mutations have about 2–3 times the risk of PDAC, while those with carriers of BRCA1 mutations have about 1.6–2.3 times the risk (17-20). PALB2, a gene that acts in concert with BRCA2, also confers additional risk (21). Other syndromes associated with familial pancreatic cancer include Familial Atypical Multiple Mole Melanoma Syndrome (FAMMM) (22,23); germline mutations in the tumor suppressor gene CDKN2A lead to heightened risk of PDAC as well as melanoma.
A recent study (24) investigated germline mutations in several genes in 515 patients with PDAC who met the definition of “familial pancreatic cancer”; that is, they were part of a family with at least one pair of affected first degree relatives. In these patients, BRCA2 mutations were identified in 3.7%; CDNK2A in 2.5%; BRCA1 in 1.2%; and PALB2 in 0.6%, for a total of 8% with mutations in any of these genes. In 201 additional patients in this study with a family history of pancreatic cancer but who did not meet the stricter criterion, a total of 3.0% had mutations in BRCA2 and 0.5% in PALB2. Mutations in ATM have been found in 1.2–3.2% of patients with familial pancreatic cancer; this gene is also involved in DNA repair and homozygous or compound heterozygous mutations are responsible for ataxia-telangiectasia. Mutations in genes related to Lynch Syndrome, also known as Hereditary Non-Polyposis Colorectal Cancer, have also been associated with increased risk of pancreatic cancer (25,26). Recent reviews summarized the existing data on the prevalence of these and other mutations in patients with familial pancreatic cancer (21) and their potential functional significance (27). Other rare genetic syndromes that increase risk of PDAC include hereditary pancreatitis, caused by mutations in PRSS1 (28); Peutz-Jeghers syndrome, caused by germline mutations in STK11 (29); and Familial Adenomatous Polyposis (FAP), caused by germline mutations in APC (30). Overall, while a positive family history confers increased risk of PDAC, prevalence of mutations in known genes is low overall. Ongoing studies are evaluating the usefulness of surveillance programs for individuals at increased risk because of family history and germline mutations.
In addition to the rare disease-causing genetic mutations discussed above, there are a number of more common genetic polymorphisms that have been found to lead to increased or decreased risk of PDAC, based on a number of genome wide association studies (GWAS) conducted by the PanScan and PanC4 groups and others in recent years. Changes in risk associated with these polymorphisms are usually small, but cumulatively, they may be important in overall risk; in addition, they provide insight into the genetic basis for the development of PDAC. A total of 16 loci have been identified and confirmed through these studies (31-34); results are summarized in a recent review (27). The most recently published study included >11,000 cases and >16,000 controls in discovery and replication sets (34). Work is ongoing to determine the functional significance of these regions to enhance understanding of their effect on risk of PDAC.
Diabetes mellitus
Diabetes mellitus has been associated with an increased risk of developing PDAC. It remains unclear whether diabetes plays a causative role in the development of pancreatic cancer, whether it is an early manifestation of pancreatic cancer, or if there is an underlying genetic or metabolic association that affects the development of both these conditions. Insulin resistance, resultant hyper insulinemia and increased circulating levels of IGF-1 are thought to inhibit apoptosis and thus promote the development of pancreatic tumors (35). It is clear that many patients with pancreatic cancer develop diabetes in the 3 or so years preceding their diagnosis, indicating that this is an early sign of PDAC (36,37); however, the precise association of risk with longer-term diabetes is unclear as studies have shown conflicting results.
An analysis from PanC4, pooling data from approximately 8,000 cases of PDAC and 14,000 controls in 15 studies, found a higher ORs for diabetes diagnosed shortly before the diagnosis of PDAC (OR =3.68, 95% CI 2.68–4.77 for <2 years and OR =10.32, 95% CI 7.48–14.23 for <1 year of diabetes). Risk was also elevated among those who had diabetes for 15–20 years prior to diagnosis of pancreatic cancer (OR =1.54, 95% CI 1.17–2.03) and for those with diabetes of ≥20 years duration (OR =1.30, 95% CI 1.03–1.63). The study also found the risk of developing PDAC was higher among diabetics using insulin than those using oral hypoglycemic drugs (38), indicating poorer control of diabetes. A meta-analysis of 35 cohort studies also found high RR for those with shorter duration of diabetes (1–4 years, RR =1.95, 95% CI 1.65–2.31); the association was less strong with longer duration of diabetes: RR =1.49 (95% CI 1.05–2.12) for those with 5–9 years’ duration, and RR =1.47 (95% CI 0.94–2.31) for ≥10 years’ duration (39). In a pooled analysis from PanScan, with 12 cohort studies (mostly different from those included in the meta-analysis), the investigators found a significantly increased risk of developing PDAC among those with shorter duration of diabetes (2–8 years, OR =1.79, 95% CI 1.25–2.55), but not among those with long standing diabetes (>9 years, OR =1.02, 95% CI 0.68–1.52) (40). All these studies point to an inverse relationship between duration of diabetes and PDAC risk; however, the risk associated with long-term diabetes remains unclear. The PACIFIC study, a case-control study with 654 cases and 697 controls from two large health plans, found significant association between the development of PDAC and a history of diabetes in a first degree relative, including offspring (OR =1.95, 95% CI 1.23–3.09), siblings (OR =1.39, 95% CI 1.05–1.82) and parents (OR =1.32, 95% CI 1.07–1.62), suggesting a common underlying genetic susceptibility to both these diseases (41). It is possible that family history of diabetes in family members of PDAC patients reflects shared environmental exposures as well as genetic factors. Insulin resistance and resultant hyperinsulinemia during the early stages of Type 2 diabetes are hypothesized to play a role. Hypoinsulinemia that eventually develops from loss of beta islet cells may be responsible for the decreased risk in long standing diabetes (42).
Allergies
The presence of allergies has consistently been found to be protective against the development of PDAC, although the mechanism remains unknown. Pooled data from 10 studies in PanC4, with >3,500 cases and >9,000 controls, showed reduced risk for developing pancreatic cancer among those with hay fever and those with allergy to animals compared to those without allergies (OR =0.74, 95% CI 0.56–0.96) and OR =0.62 (95% CI 0.41–0.94), respectively (43). Findings were similar in an earlier meta-analysis of 14 studies (4 cohort and 10 case-control, 4 also included in the pooled analysis): RR for allergies related to atopic conditions (such as rhinitis, asthma, eczema, animals) was 0.71 (95% CI 0.64–0.80) (44). Other case-control studies not included in the these analyses corroborated these results: one (45) reported a statistically significant association with history of hay fever (OR =0.68, 95% CI 0.52–0.90); and the other (46) reported association with symptoms of nasal allergies (OR =0.73, 95% CI 0.60–0.87). The association with asthma has been less well-established: while some studies have found no association (43-45), a recent new study and meta-analysis of 10 case-control studies reported significantly reduced risk associated with history of asthma (meta-analysis OR =0.73, 95% CI 0.59–0.89) (46). While it has been hypothesized that presence of allergies is a sign of a stronger immune system that can lead to reduced cancer risk (44), there is little evidence of a direct mechanism. A prospective study of pre-diagnostic levels of serum IgE, indicating immune response to specific antigens, did not find this biomarker to be related to risk (47).
Alcohol
Studies have found an association between heavy alcohol consumption and pancreatic cancer. Alcohol and its metabolites, especially acetaldehyde, may potentiate the effects of other carcinogens, especially those in tobacco, and thus accelerate the development of pancreatic cancer. Heavy alcohol intake may also lead to the development of chronic pancreatitis, which has also been associated with an increased risk of pancreatic cancer (48), through the production of reactive oxygen species (4). A pooled analysis of 19 prospective cohort studies found high alcohol intake (>24 g/day or 2 drinks/day) was associated with an increased risk of pancreatic cancer in men (RR =1.18; 95% CI 1.00–1.39; P=0.045), but no association in women. Low (0–12 g/day) and moderate (>12–24 g/day) alcohol intake were not associated with increased risk (49). Data from the Pancreas Cancer Cohort Consortium (PanScan) had similar results: there was an increase in pancreas cancer risk among men consuming 45 or more grams of alcohol from liquor per day (OR =2.23, 95% CI 1.02–4.87, compared to 0 g/day of alcohol from liquor, P-trend =0.12), but not among women (OR =1.35, 95% CI 0.63–2.87, for 30 or more g/day of alcohol from liquor, compared to none) Lower levels of consumption were not associated with risk (50). Case-control studies had similar findings: in an analysis of 10 studies, there was no increased risk noted for moderate consumption, while the OR for consumption of >9 drinks per day was 1.60 (95% CI 1.16–2.22); in contrast to the cohort studies, there were no differences between men and women (51). Overall, consistent evidence indicates that while heavy alcohol consumption increases risk of PDAC, moderate consumption does not.
Physical activity
Increased physical activity has been weakly linked to lowered risk of developing PDAC. The risk reduction is attributed to decreased insulin resistance higher adiponectin levels and reduced chronic inflammation (52). Two large meta-analyses were recently published: one (53) included 19 cohort studies and 7 case-control studies; and the other (54) included 23 cohort studies and 7 case-control studies, with a good deal of overlap of the studies included. Farris et al. (53) found a modest 11% risk reduction in the development of PDAC, comparing the highest and lowest levels of leisure time physical activity (OR =0.89, 95% CI 0.82–0.96). Statistically significant results were found only in case-control studies (RR =0.69, 95% CI 0.59–0.81); in cohort studies, the combined OR =0.96 (95% CI 0.91–1.02). While only a small number of studies (n=4) enrolled subjects with a median age <50, the strongest protective effect was observed in these studies (OR =0.61, 95% CI 0.50–0.75) (53). Similar results were found in the study led by Behrens (54), with an overall OR =0.93 (95% CI 0.88–0.98) for high levels of leisure or occupational physical activity; again, results were stronger in case-control studies than in cohort studies. This study analyzed results according to smoking status and BMI, finding that these exposures did not appear to modify the association of physical activity and risk. They did find that consistent physical activity over time was more beneficial than recent or long-ago activity, with an OR =0.86 (95% CI 0.76–0.97) in cohort studies and OR =0.74 (95% CI 0.61–0.90) in case-control studies. While physical activity is linked to other risk factors for PDAC, high BMI and diabetes, low physical activity itself appears to be only weakly associated with risk.
Oral health
Poor oral health has been associated with various cancers, and recent studies have investigated various aspects of oral health in relation to risk of PDAC. A meta-analysis of 8 studies was recently published (55), focused on periodontitis (in 6 cohort studies) and tooth loss (in 3 cohort studies and one case-control study) as exposures. The studies varied in the method for determining periodontal disease and tooth loss and the categorization of these variables. The combined RR for periodontal disease was 1.74 (95% CI 1.14–2.15), and for severe tooth loss, RR =1.54 (95% CI 1.16–2.05). Variations in the salivary microbiota have been noted between people with pancreas cancer and healthy controls (56), suggesting that salivary biomarkers might be of use in ascertaining the risk or presence of pancreas cancer. Study of oral health and risk is at an early stage but seems likely to add to understanding of the etiology of PDAC.
Conclusions
A number of environmental and genetic factors have been identified that influence an individual’s risk for the development of PDAC. The most important modifiable risk factor is cigarette smoking; maintaining a normal body weight and prevention of diabetes are also likely to reduce risk. Our work at MSKCC has focused in part on identification and surveillance of individuals at increased risk because of their family history and/or genetic background; several other centers have developed similar registries. It is still not clear from our work and that of others whether surveillance in high risk individuals is worthwhile, since PDAC is rare and surveillance methods are imperfect. We hope that the continued search for unique biomarkers will be successful and will eventually lead to earlier diagnosis of this disease even in asymptomatic individuals, leading to improved overall survival.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Globocan. GLOBOCAN 2012 v1.0: estimated cancer incidence, mortality and prevalence worldwide in 2012. International Agency for Research on Cancer 2012.
- The Surveillance E, and End Results (SEER) Program of the National Cancer Institute. Cancer Stat Facts: Pancreas Cancer. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html
- Society AC. Pancreatic Cancer Survival Rates, by Stage. Available online: https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html
- Duell EJ. Epidemiology and potential mechanisms of tobacco smoking and heavy alcohol consumption in pancreatic cancer. Mol Carcinog 2012;51:40-52. [Crossref] [PubMed]
- Bosetti C, Lucenteforte E, Silverman DT, et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol 2012;23:1880-8. [Crossref] [PubMed]
- Lynch SM, Vrieling A, Lubin JH, et al. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol 2009;170:403-13. [Crossref] [PubMed]
- Zou L, Zhong R, Shen N, et al. Non-linear dose-response relationship between cigarette smoking and pancreatic cancer risk: evidence from a meta-analysis of 42 observational studies. Eur J Cancer 2014;50:193-203. [Crossref] [PubMed]
- Michaud DS. Obesity and Pancreatic Cancer. Recent Results Cancer Res 2016;208:95-105. [Crossref] [PubMed]
- Aune D, Greenwood DC, Chan DS, et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol 2012;23:843-52. [Crossref] [PubMed]
- Wang J, Yang DL, Chen ZZ, et al. Associations of body mass index with cancer incidence among populations, genders, and menopausal status: A systematic review and meta-analysis. Cancer Epidemiol 2016;42:1-8. [Crossref] [PubMed]
- Arslan AA, Helzlsouer KJ, Kooperberg C, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med 2010;170:791-802. [Crossref] [PubMed]
- Genkinger JM, Kitahara CM, Bernstein L, et al. Central adiposity, obesity during early adulthood, and pancreatic cancer mortality in a pooled analysis of cohort studies. Ann Oncol 2015;26:2257-66. [Crossref] [PubMed]
- Hruban RH, Canto MI, Goggins M, et al. Update on familial pancreatic cancer. Adv Surg 2010;44:293-311. [Crossref] [PubMed]
- Jacobs EJ, Chanock SJ, Fuchs CS, et al. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Int J Cancer 2010;127:1421-8. [Crossref] [PubMed]
- Permuth-Wey J, Egan KM. Family history is a significant risk factor for pancreatic cancer: results from a systematic review and meta-analysis. Fam Cancer 2009;8:109-17. [Crossref] [PubMed]
- Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res 2004;64:2634-8. [Crossref] [PubMed]
- Mocci E, Milne RL, Mendez-Villamil EY, et al. Risk of pancreatic cancer in breast cancer families from the breast cancer family registry. Cancer Epidemiol Biomarkers Prev 2013;22:803-11. [Crossref] [PubMed]
- Thompson D, Easton DF. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst 2002;94:1358-65. [Crossref] [PubMed]
- Cancer risks in BRCA2 mutation carriers. The Breast Cancer Linkage Consortium. J Natl Cancer Inst 1999;91:1310-6. [Crossref] [PubMed]
- Iqbal J, Ragone A, Lubinski J, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer 2012;107:2005-9. [Crossref] [PubMed]
- Petersen GM. Familial pancreatic cancer. Semin Oncol 2016;43:548-53. [Crossref] [PubMed]
- Bergman W, Watson P, de Jong J, et al. Systemic cancer and the FAMMM syndrome. Br J Cancer 1990;61:932-6. [Crossref] [PubMed]
- Goldstein AM. Familial melanoma, pancreatic cancer and germline CDKN2A mutations. Hum Mutat 2004;23:630. [Crossref] [PubMed]
- Zhen DB, Rabe KG, Gallinger S, et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet Med 2015;17:569-77. [Crossref] [PubMed]
- Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA 2009;302:1790-5. [Crossref] [PubMed]
- Grant RC, Selander I, Connor AA, et al. Prevalence of germline mutations in cancer predisposition genes in patients with pancreatic cancer. Gastroenterology 2015;148:556-64. [Crossref] [PubMed]
- Amundadottir LT. Pancreatic Cancer Genetics. Int J Biol Sci 2016;12:314-25. [Crossref] [PubMed]
- Rebours V, Boutron-Ruault MC, Schnee M, et al. The natural history of hereditary pancreatitis: a national series. Gut 2009;58:97-103. [Crossref] [PubMed]
- Giardiello FM, Welsh SB, Hamilton SR, et al. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med 1987;316:1511-4. [Crossref] [PubMed]
- Giardiello FM, Offerhaus GJ, Lee DH, et al. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut 1993;34:1394-6. [Crossref] [PubMed]
- Petersen GM, Amundadottir L, Fuchs CS, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet 2010;42:224-8. [Crossref] [PubMed]
- Wolpin BM, Rizzato C, Kraft P, et al. Genome-wide association study identifies multiple susceptibility loci for pancreatic cancer. Nat Genet 2014;46:994-1000. [Crossref] [PubMed]
- Childs EJ, Mocci E, Campa D, et al. Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat Genet 2015;47:911-6. [Crossref] [PubMed]
- Zhang M, Wang Z, Obazee O, et al. Three new pancreatic cancer susceptibility signals identified on chromosomes 1q32.1, 5p15.33 and 8q24.21. Oncotarget 2016;7:66328-43. [PubMed]
- Bao B, Wang Z, Li Y, et al. The complexities of obesity and diabetes with the development and progression of pancreatic cancer. Biochim Biophys Acta 2011;1815:135-46.
- Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology 2008;134:95-101. [Crossref] [PubMed]
- Pannala R, Leirness JB, Bamlet WR, et al. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008;134:981-7. [Crossref] [PubMed]
- Bosetti C, Rosato V, Li D, et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: an analysis from the International Pancreatic Cancer Case-Control Consortium. Ann Oncol 2014;25:2065-72. [Crossref] [PubMed]
- Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer 2011;47:1928-37. [Crossref] [PubMed]
- Elena JW, Steplowski E, Yu K, et al. Diabetes and risk of pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Cancer Causes Control 2013;24:13-25. [Crossref] [PubMed]
- Austin MA, Kuo E, Van Den Eeden SK, et al. Family history of diabetes and pancreatic cancer as risk factors for pancreatic cancer: the PACIFIC study. Cancer Epidemiol Biomarkers Prev 2013;22:1913-7. [Crossref] [PubMed]
- Li D. Diabetes and pancreatic cancer. Mol Carcinog 2012;51:64-74. [Crossref] [PubMed]
- Olson SH, Hsu M, Satagopan JM, et al. Allergies and risk of pancreatic cancer: a pooled analysis from the pancreatic cancer case-control consortium. Am J Epidemiol 2013;178:691-700. [Crossref] [PubMed]
- Gandini S, Lowenfels AB, Jaffee EM, et al. Allergies and the risk of pancreatic cancer: a meta-analysis with review of epidemiology and biological mechanisms. Cancer Epidemiol Biomarkers Prev 2005;14:1908-16. [Crossref] [PubMed]
- Cotterchio M, Lowcock E, Hudson TJ, et al. Association between allergies and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2014. [Crossref] [PubMed]
- Gomez-Rubio P, Zock JP, Rava M, et al. Reduced risk of pancreatic cancer associated with asthma and nasal allergies. Gut 2017;66:314-22. [Crossref] [PubMed]
- Olson SH, Hsu M, Wiemels JL, et al. Serum immunoglobulin e and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev 2014;23:1414-20. [Crossref] [PubMed]
- Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 2012;23:2964-70. [Crossref] [PubMed]
- Wang YT, Gou YW, Jin WW, et al. Association between alcohol intake and the risk of pancreatic cancer: a dose-response meta-analysis of cohort studies. BMC Cancer 2016;16:212. [Crossref] [PubMed]
- Michaud DS, Vrieling A, Jiao L, et al. Alcohol intake and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium (PanScan). Cancer Causes Control 2010;21:1213-25. [Crossref] [PubMed]
- Lucenteforte E, La Vecchia C, Silverman D, et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 2012;23:374-82. [Crossref] [PubMed]
- Bao Y, Giovannucci EL, Kraft P, et al. A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. J Natl Cancer Inst 2013;105:95-103. [Crossref] [PubMed]
- Farris MS, Mosli MH, McFadden AA, et al. The Association between Leisure Time Physical Activity and Pancreatic Cancer Risk in Adults: A Systematic Review and Meta-analysis. Cancer Epidemiol Biomarkers Prev 2015;24:1462-73. [Crossref] [PubMed]
- Behrens G, Jochem C, Schmid D, et al. Physical activity and risk of pancreatic cancer: a systematic review and meta-analysis. Eur J Epidemiol 2015;30:279-98. [Crossref] [PubMed]
- Maisonneuve P, Amar S, Lowenfels AB. Periodontal disease, edentulism, and pancreatic cancer: a meta-analysis. Ann Oncol 2017;28:985-95. [PubMed]
- Fan X, Alekseyenko AV, Wu J, et al. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut 2016. [Epub ahead of print]. [Crossref] [PubMed]