Radiation therapy for WHO grade I meningioma
Overview
Meningiomas are the most common type of extraaxial primary brain tumor, representing a third of all primary central nervous system (CNS) neoplasms (1). They were first described by Cushing as a group of tumors arising from the dural coverings of the CNS, and they can arise anywhere dura is found (2). The prevalence of pathologically-confirmed meningioma is estimated to be approximately 97.5/100,000 in the United States, with 170,000 currently diagnosed patients (3). Approximately 75–90% of these tumors are WHO Grade I lesions that typically progress slowly.
Observation reveals that growth rates and patterns may vary among patients, even when all are WHO grade I and benign (4). Disease progression or symptomatic presentation may ultimately necessitate intervention in many patients with WHO grade I meningioma. Significant morbidity may occur due to mass effect or infiltration of adjacent brain and cranial nerves. Though gross total surgical resection (GTR) has long been the primary treatment for meningioma, it is not always possible or may be associated with considerable morbidity. Thus, radiation therapy has long had a role as adjunct therapy after subtotal resection (STR) for recurrence. In addition, radiation therapy has a role in the primary treatment of image defined meningioma especially in surgically inaccessible areas or for candidates who are medically inoperable or do not wish to undergo surgery.
As we continue to accrue long-term follow up for patients treated with radiation in conjunction with, or as an alternative to surgery, it is apparent that radiation therapy represents a valuable tool in the treatment of the various presentations of WHO grade I meningioma. Multiple treatment techniques including stereotactic radiosurgery (SRS), fractionated external beam radiation therapy, and particle therapy using protons have been used successfully for the treatment of these tumors. The use of radiation for the treatment of WHO grade I meningiomas will be reviewed here.
Radiation therapy after STR
When possible, a gross total resection (GTR) remains the cornerstone of definitive management of WHO Grade 1 meningioma. Since the 1950’s, the extent of resection of meningioma has commonly been described by the Simpson grading scale (5). Simpson grading depends on intraoperative observations and categorizes resection quality along a scale from 1–5. A Simpson 1 resection removes all tumor, associated dural attachments and involved bone, whereas a Simpson 5 resection is simple decompression only. In most studies, a Simpson grade 1–3 resection is considered a GTR (5,6). Successfully achieving GTR has been an important prognostic factor in patients with all grades of meningioma (6), with STR resulting in increased risk of recurrence (7,8). In approximately 30% of cases, GTR is impossible owing to tumor location or proximity to neurologic or vascular structures (7,9,10); this is especially true for meningiomas involving the sphenoid ridge, posterior fossa, parasellar region, and optic nerve sheath (10,11).
Numerous single institution studies with extended follow-up of benign meningiomas suggest that the 5-year local progression rate after STR alone may range from 37–62%, and 10-year local progression may be as high as 52–100% (Table 1) (7,12-17). There remains controversy regarding whether patients with meningioma after STR should undergo active surveillance or radiation therapy. Some studies have suggested that small amounts of residual tumor can be observed with high PFS rates. For example, a 2010 analysis by Sughrue et al. found a 81% 5 y PFS rate after Simpson 4 resection (18). Thus, patients with advanced age or multiple comorbidities and small residual tumor may not recur, or perhaps more importantly, may not grow enough to cause symptoms.
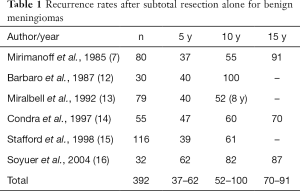
Full table
However, others argue that especially in young patients with tumor locations that are likely to become symptomatic, adjuvant radiation therapy is indicated. Multiple observational studies have demonstrated the benefit of fractionated EBRT in the adjuvant setting following STR of benign meningioma. Across these series, 74–96% of patients remained progression-free on long-term follow-up (generally 10 years) after GTR alone. After STR without adjuvant treatment, PFS ranges dropped to 18–52%. Adjuvant radiation prevented local control failures after STR with results ranging from 68–100% of patients free from progression at extended follow-up (Table 2).

Full table
Furthermore, certain series suggest that there may be a survival benefit to adjuvant radiation therapy after STR. Condra and colleagues noted that STR significantly affected cause specific survival (CSS) in patients with benign meningioma. They reported that the 15-year CSS dropped from 88% to 51% when comparing GTR to STR in patients with resected grade 1 lesions. They noted that the addition of radiation therapy after STR reversed this drop in survival, with a return to a 15 y CSS of 86% in patients treated with EBRT following STR (14). Of note, this work likely included patients that would now be categorized as WHO grade II, thus potentially overestimating the true benefit of radiation in the lowest risk patients. However, in a modern single-pathologist analysis of 236 grade 1 lesions that utilized WHO 2007 classifications, STR was found to be a significant factor in reduced PFS (HR 4.18, P=0.007), and overall survival (OS) (HR 2.69, P=0.009). The median age in this series was 56 years and median follow up was 75 months (24). Of note, this study defined GTR as Simpson 1 or 2 resections, and considered Simpson 3 resections as STR.
Though most of the early series utilized standard external beam radiation therapy with margins added, more recent series have used improved imaging and targeting capabilities with stereotactic localization to successfully treat skull base tumors with higher precision. These studies show excellent local control rates with the use of fractionated stereotactic radiotherapy (FSRT) or SRS after STR (Table 3). Although longer-term follow up will be required given the risk of late recurrences, the initial results in over 1,100 patients across multiple reports with 3, 5 and 10 years follow up show LC rates in excess of 92–100% at five years, and 88–95% at ten years. Patients in these studies were generally treated using fractions of 1.8–2 Gy to a total dose of 50.4–54 Gy for FSRT and to 13–16 Gy in a single fraction for SRS. There is accruing long-term evidence that EBRT, FSRT and SRS are attractive tools for management of patients after STR or with surgically difficult presentations.

Full table
Radiation therapy as primary treatment
Radiation therapy has emerged as a viable alternative when the patient has a surgically inaccessible tumor, is medically inoperable, or prefers radiation therapy over surgical intervention. Initially, radiation therapy was used in tumors that were not surgically accessible. For instance, though initially surgical resection was pursued for optic nerve sheath meningiomas, high rates of blindness were associated with optic nerve infarction during surgery (33,34). Thus, standard fractionated external beam radiation therapy to 50–54 Gy was utilized. Published data suggests that treated tumors regress or remain stable in greater than 90% of cases after primary radiotherapy alone (34-37), and that vision can be maintained after irradiation.
Similarly, surgical resection of cavernous sinus meningioma was often associated with cranial neuropathies and thus multiple series outline good outcomes after radiation therapy alone, either standard fractionated external beam radiation therapy or stereotactic radiosurgery (28,38-43). These experiences led to the successful treatment of meningioma with radiation therapy in other locations and for patients who were medically inoperable or preferred radiation therapy over surgical resection.
The increase in use of SRS has also been influenced by the increase in the number of radiographically detected, clinically occult lesions being discovered in the era of widespread medical imaging (5,44). Many of these asymptomatic lesions are small enough for active surveillance or treatment with SRS. Statistically, most of these incidental lesions are be WHO grade I meningiomas, and success in treating imaging-defined meningioma suggests that radiotherapy may be effective in a majority of cases. Given the slow growth of these tumors and the possibility of late recurrence, longer term follow-up is warranted, but the evidence supporting the use of primary radiotherapy in imaging defined meningioma is accumulating. A number of series reporting patients treated with FSRT alone or GKRS alone show similarly excellent LC rates, ranging from 89–99% at 5 years and 79–97% at 10 years (Table 4). One of the largest studies published is a retrospective analysis of more than 4,500 patients with a median follow-up of 63 months. It included approximately 3000 imaging-defined meningiomas treated with radiosurgery alone and tumor control, defined as a reduction in volume or stable size, was 92.5% (50).
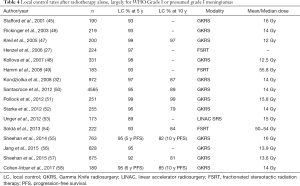
Full table
Stereotactic radiosurgery versus fractionated external beam radiation therapy
Though initial experience with radiation therapy included standard external beam radiation therapy, in an effort to minimize toxicity including long-term effects clinicians began using more precisely targeted approaches. This was facilitated by advances in imaging including the more prevalent use of magnetic resonance imaging (MRI) and in stereotactic delivery including cobalt-60 machines, linear accelerators, robotic linear accelerators, and proton therapy. Throughout the 1990s, reports on standard external beam radiation therapy for meningioma were published. However, by the 2000s, most reports were on FSRT and SRS.
Typically SRS is reserved for tumors that are less than 3 cm in diameter or about 10 cm3 in volume. Multiple studies have suggested that efficacy is based on tumor size. For instance, DiBiase and colleagues reported a 5-year disease free survival of 91.9% for patients with meningiomas less than 10 cm3 as opposed to 68% for larger tumors (59). In addition, toxicity is quite dependent on size. Pollack and colleagues reported on a 22-year experience with SRS for presumed meningioma, with complication rates of 4.8% for patients whose tumors were less than 3.2 cm3 and 22.6% for patients whose tumors were greater than 9.6 cm3 (51). These complications included cranial nerve deficits, headaches, hemiparesis, new/worsened seizure, cyst formation and stroke.
For larger tumors or those that are close to dose limiting normal structures such as the optic nerves and chiasm, fractionated radiation therapy has been most used. However, there are increasing reports of multisession stereotactic radiation therapy utilizing many of the same platforms used for SRS (53,60-63). These studies demonstrate comparable local control compared to single fraction treatment and its proponents argue that side effects may be fewer.
For instance, Unger and colleagues reported on 173 patients with meningiomas where 56% underwent single fraction radiosurgery with Gamma Knife and the remainder received multisession radiation therapy over generally 2 to 5 fractions with CyberKnife. The median dose for SRS was 15 Gy and the usual regimen was 25 Gy in 5 fractions for multisession stereotactic radiation therapy. Two-year actuarial risk of symptomatic edema was 3.2% for multisession stereotactic radiation therapy, and 12.5% for SRS. Tumor size greater than 4.9 cm3 was also a significant predictor of symptomatic edema (53). However, recently Conti and colleagues have published on 229 patients treated with single or multisession radiosurgery with Cyberknife and found that tumor volume, tight brain/tumor interface, non-basal tumor location and atypical histology were associated with post-treatment edema. The treatment modality (single versus multisession) was not significantly associated with post-treatment edema (61).
As we continue to accrue follow up and data on the long-term efficacy of multisession radiosurgery, clinicians continue to use techniques for more precise delivery of standard fractionated radiation therapy (25-30). This includes utilization of stereotactic frames and/or masks, skull based fiducials for localization and more recently, onboard imaging with CT scans.
Particle therapy in the form of proton therapy has also been reported for the treatment of patients with benign meningioma. The unique property of the Bragg peak that prevents exit dose reduces the integral radiation dose to the normal brain, temporal lobes, hippocampi, cochleae, brainstem, and pituitary. Because of this dose reduction, protons are predicted to lower the risk of neurocognitive decline and excess risk of second tumors (1.3 vs. 2.8 per 10,000 patients per year; P<0.002) (64). Based on several proton therapy series of incompletely resected or recurrent benign meningioma, the five- or ten-year LC is 88–100% with acceptable toxicity (37,65-67).
Late effects
As illustrated above, the large majority of patients with benign meningioma will achieve long-term tumor control. Thus, the toxicity and late effects associated with treatment are important to consider. Many have questioned whether radiation causes benign meningiomas to transform to a higher grade. However, malignant transformation has not been definitively linked to radiation therapy. It is difficult to determine the rate of malignant transformation with and without radiation therapy since for a subgroup of recurrent or progressive meningiomas, the natural history is to transform to a higher grade (68,69) Regardless, transformation after treatment for benign meningioma is relatively rare. Pollock and colleagues found that 2.2% of patients with meningioma with at least 5 years imaging follow up (median follow up 9.2 years) had transformation to a higher grade after radiosurgery (70).
Aside from malignant transformation, radiation therapy is also associated with secondary tumors. Estimates vary, however perhaps best illustrated by long-term follow up of patients treated with external beam radiation therapy for pituitary adenomas. Minniti and colleagues reported on 426 patients with pituitary adenoma treated with surgery and external beam radiation therapy with 5,749 person years. They found a risk of second brain tumor at 20 years of 2.4%. Of the 11 second tumors, 5 were meningiomas (71). Breen and colleagues reported on 120 patients with median follow up of 9 years, and found 2.7% developed radiation-induced neoplasms at 10 and 30 years (72). This rate is likely to be lower with modern techniques with smaller radiation fields and/or with radiosurgery. Pollock and colleagues recently reviewed 1,837 patients with 11,264 patient-years of follow-up after SRS for benign tumors and found that the 15-year risk of developing a radiation-induced tumor was 0.0% (95% confidence interval 0.0–2.8%) (70). There have been very few reported cases of radiation-induced tumor after SRS.
In addition, patients receiving radiation therapy to the brain are at risk for other late effects including neurocognitive decline and hypopituitarism. The impact of radiation therapy on long term neurocognitive function is not well described for patients with meningioma. It has been shown that neurocognitive function in patients with benign or low-grade tumors is related radiation dose to the bilateral hippocampi (73). In addition, other areas of the brain are also likely to be involved with neurocognition. The risk of hypopituitarism is likewise related to the dose received (74). Thus, continued efforts to minimize radiation dose and extent to normal brain and pituitary are likely to improve the risk of late effects but not completely eliminate them. Ongoing trials such as the ROAM/EORTC-1308 trial for radiation versus observation following surgical resection of atypical meningioma may help to characterize the neurocognitive outcomes for our meningioma patients in the future (75).
Conclusions
Overall, radiation therapy for benign meningioma is associated with excellent local control rates with minimal toxicity. Late effects of radiation therapy appear rare but possible, and further study of their true prevalence would be helpful to determining the risks and benefits of treatment. Future advances will concentrate on advanced imaging and molecular testing of pathology that can help to further differentiate the risk of recurrence. This information could help us determine not only which patients could be observed, but also which patients may need a higher dose or extent of radiation therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Whittle IR, Smith C, Navoo P, et al. Meningiomas. Lancet 2004;363:1535-43. [Crossref] [PubMed]
- Cushing H. Meningiomas: their classification, regional behaviour, life history, and surgical end results. New York,: Hafner Pub. Co.; 1962.
- Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol 2010;99:307-14. [Crossref] [PubMed]
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97-109. [Crossref] [PubMed]
- Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 1957;20:22-39. [Crossref] [PubMed]
- Gallagher MJ, Jenkinson MD, Brodbelt AR, et al. WHO grade 1 meningioma recurrence: Are location and Simpson grade still relevant? Clin Neurol Neurosurg 2016;141:117-21. [Crossref] [PubMed]
- Mirimanoff RO, Dosoretz DE, Linggood RM, et al. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg 1985;62:18-24. [Crossref] [PubMed]
- Black PM. Meningiomas. Neurosurgery 1993;32:643-57. [Crossref] [PubMed]
- Pollock BE, Stafford SL, Link MJ. Gamma knife radiosurgery for skull base meningiomas. Neurosurg Clin N Am 2000;11:659-66. [PubMed]
- Levine ZT, Buchanan RI, Sekhar LN, et al. Proposed grading system to predict the extent of resection and outcomes for cranial base meningiomas. Neurosurgery 1999;45:221-30. [Crossref] [PubMed]
- Mathiesen T, Lindquist C, Kihlstrom L, et al. Recurrence of cranial base meningiomas. Neurosurgery 1996;39:2-7; discussion 8-9. [Crossref] [PubMed]
- Barbaro NM, Gutin PH, Wilson CB, et al. Radiation therapy in the treatment of partially resected meningiomas. Neurosurgery 1987;20:525-8. [Crossref] [PubMed]
- Miralbell R, Linggood RM, de la Monte S, et al. The role of radiotherapy in the treatment of subtotally resected benign meningiomas. J Neurooncol 1992;13:157-64. [Crossref] [PubMed]
- Condra KS, Buatti JM, Mendenhall WM, et al. Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys 1997;39:427-36. [Crossref] [PubMed]
- Stafford SL, Perry A, Suman VJ, et al. Primarily resected meningiomas: outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin Proc 1998;73:936-42. [Crossref] [PubMed]
- Soyuer S, Chang EL, Selek U, et al. Radiotherapy after surgery for benign cerebral meningioma. Radiother Oncol 2004;71:85-90. [Crossref] [PubMed]
- Wara WM, Sheline GE, Newman H, et al. Radiation therapy of meningiomas. Am J Roentgenol Radium Ther Nucl Med 1975;123:453-8. [Crossref] [PubMed]
- Sughrue ME, Rutkowski MJ, Aranda D, et al. Treatment decision making based on the published natural history and growth rate of small meningiomas. J Neurosurg 2010;113:1036-42. [Crossref] [PubMed]
- Adegbite AB, Khan MI, Paine KW, et al. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg 1983;58:51-6. [Crossref] [PubMed]
- Taylor BW Jr, Marcus RB Jr, Friedman WA, et al. The meningioma controversy: postoperative radiation therapy. Int J Radiat Oncol Biol Phys 1988;15:299-304. [Crossref] [PubMed]
- Glaholm J, Bloom HJ, Crow JH. The role of radiotherapy in the management of intracranial meningiomas: the Royal Marsden Hospital experience with 186 patients. Int J Radiat Oncol Biol Phys 1990;18:755-61. [Crossref] [PubMed]
- Peele KA, Kennerdell JS, Maroon JC, et al. The role of postoperative irradiation in the management of sphenoid wing meningiomas. A preliminary report. Ophthalmology 1996;103:1761-6; discussion 6-7.
- Ohba S, Kobayashi M, Horiguchi T, et al. Long-term surgical outcome and biological prognostic factors in patients with skull base meningiomas. J Neurosurg 2011;114:1278-87. [Crossref] [PubMed]
- Jensen R, Lee J. Predicting outcomes of patients with intracranial meningiomas using molecular markers of hypoxia, vascularity, and proliferation. Neurosurgery 2012;71:146-56. [Crossref] [PubMed]
- Jalali R, Loughrey C, Baumert B, et al. High precision focused irradiation in the form of fractionated stereotactic conformal radiotherapy (SCRT) for benign meningiomas predominantly in the skull base location. Clin Oncol (R Coll Radiol) 2002;14:103-9. [Crossref] [PubMed]
- Selch MT, Ahn E, Laskari A, et al. Stereotactic radiotherapy for treatment of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys 2004;59:101-11. [Crossref] [PubMed]
- Henzel M, Gross MW, Hamm K, et al. Significant Tumorvolume Reduction Of Meningiomas Afterstereotactic Radiotherapy: Results Of A Prospective Multicenter Study. Neurosurgery 2006;59:1188-94. [Crossref] [PubMed]
- Metellus P, Batra S, Karkar S, et al. Fractionated conformal radiotherapy in the management of cavernous sinus meningiomas: long-term functional outcome and tumor control at a single institution. Int J Radiat Oncol Biol Phys 2010;78:836-43. [Crossref] [PubMed]
- Tanzler E, Morris CG, Kirwan JM, et al. Outcomes of WHO Grade I meningiomas receiving definitive or postoperative radiotherapy. Int J Radiat Oncol Biol Phys 2011;79:508-13. [Crossref] [PubMed]
- Fokas E, Henzel M, Surber G, et al. Stereotactic radiation therapy for benign meningioma: long-term outcome in 318 patients. Int J Radiat Oncol Biol Phys 2014;89:569-75. [Crossref] [PubMed]
- Davidson L, Fishback D, Russin JJ, et al. Postoperative Gamma Knife surgery for benign meningiomas of the cranial base. Neurosurg Focus 2007;23:E6. [Crossref] [PubMed]
- Kondziolka D, Mathieu D, Lunsford LD, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery 2008;62:53-8; discussion 8-60. [Crossref] [PubMed]
- Turbin RE, Thompson CR, Kennerdell JS, et al. A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy. Ophthalmology 2002;109:890-9; discussion 9-900. [Crossref] [PubMed]
- Andrews DW, Faroozan R, Yang BP, et al. Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgery. Neurosurgery 2002;51:890-902; discussion 3-4. [PubMed]
- Becker G, Jeremic B, Pitz S, et al. Stereotactic fractionated radiotherapy in patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys 2002;54:1422-9. [Crossref] [PubMed]
- Milker-Zabel S, Huber P, Schlegel W, et al. Fractionated stereotactic radiation therapy in the management of primary optic nerve sheath meningiomas. J Neurooncol 2009;94:419-24. [Crossref] [PubMed]
- Arvold ND, Lessell S, Bussiere M, et al. Visual outcome and tumor control after conformal radiotherapy for patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys 2009;75:1166-72. [Crossref] [PubMed]
- Lee JY, Niranjan A, McInerney J, et al. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg 2002;97:65-72. [Crossref] [PubMed]
- dos Santos MA, de Salcedo JB, Gutierrez Diaz JA, et al. Long-term outcomes of stereotactic radiosurgery for treatment of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys 2011;81:1436-41. [Crossref] [PubMed]
- Nicolato A, Foroni R, Alessandrini F, et al. The role of Gamma Knife radiosurgery in the management of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys 2002;53:992-1000. [Crossref] [PubMed]
- Hasegawa T, Kida Y, Yoshimoto M, et al. Long-term outcomes of Gamma Knife surgery for cavernous sinus meningioma. J Neurosurg 2007;107:745-51. [Crossref] [PubMed]
- Skeie BS, Enger PO, Skeie GO, et al. Gamma knife surgery of meningiomas involving the cavernous sinus: long-term follow-up of 100 patients. Neurosurgery 2010;66:661-8; discussion 8-9. [Crossref] [PubMed]
- Dufour H, Muracciole X, Metellus P, et al. Long-term tumor control and functional outcome in patients with cavernous sinus meningiomas treated by radiotherapy with or without previous surgery: is there an alternative to aggressive tumor removal? Neurosurgery 2001;48:285-94; discussion 94-6. [PubMed]
- Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med 2007;357:1821-8. [Crossref] [PubMed]
- Stafford SL, Pollock BE, Foote RL, et al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery 2001;49:1029-37; discussion 37-8. [PubMed]
- Flickinger JC, Kondziolka D, Maitz AH, et al. Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. Int J Radiat Oncol Biol Phys 2003;56:801-6. [Crossref] [PubMed]
- Kreil W, Luggin J, Fuchs I, et al. Long term experience of gamma knife radiosurgery for benign skull base meningiomas. J Neurol Neurosurg Psychiatry 2005;76:1425-30. [Crossref] [PubMed]
- Kollová A, Liscak R, Novotny J Jr, et al. Gamma Knife surgery for benign meningioma. J Neurosurg 2007;107:325-36. [Crossref] [PubMed]
- Hamm K, Henzel M, Gross MW, et al. Radiosurgery/stereotactic radiotherapy in the therapeutical concept for skull base meningiomas. Zentralbl Neurochir 2008;69:14-21. [Crossref] [PubMed]
- Santacroce A, Walier M, Regis J, et al. Long-term tumor control of benign intracranial meningiomas after radiosurgery in a series of 4565 patients. Neurosurgery 2012;70:32-9; discussion 9. [Crossref] [PubMed]
- Pollock BE, Stafford SL, Link MJ, et al. Single-fraction radiosurgery for presumed intracranial meningiomas: efficacy and complications from a 22-year experience. Int J Radiat Oncol Biol Phys 2012;83:1414-8. [Crossref] [PubMed]
- Starke RM, Williams BJ, Hiles C, et al. Gamma knife surgery for skull base meningiomas. J Neurosurg 2012;116:588-97. [Crossref] [PubMed]
- Unger KR, Lominska CE, Chanyasulkit J, et al. Risk factors for posttreatment edema in patients treated with stereotactic radiosurgery for meningiomas. Neurosurgery 2012;70:639-45. [Crossref] [PubMed]
- Soldà F, Wharram B, De Ieso PB, et al. Long-term efficacy of fractionated radiotherapy for benign meningiomas. Radiother Oncol 2013;109:330-4. [Crossref] [PubMed]
- Sheehan JP, Starke RM, Kano H, et al. Gamma Knife radiosurgery for sellar and parasellar meningiomas: a multicenter study. J Neurosurg 2014;120:1268-77. [Crossref] [PubMed]
- Jang CK, Jung HH, Chang JH, et al. Long-Term Results of Gamma Knife Radiosurgery for Intracranial Meningioma. Brain Tumor Res Treat 2015;3:103-7. [Crossref] [PubMed]
- Sheehan JP, Starke RM, Kano H, et al. Gamma Knife radiosurgery for posterior fossa meningiomas: a multicenter study. J Neurosurg 2015;122:1479-89. [Crossref] [PubMed]
- Cohen-Inbar O, Tata A, Moosa S, et al. Stereotactic radiosurgery in the treatment of parasellar meningiomas: long-term volumetric evaluation. J Neurosurg 2017.1-11. [PubMed]
- DiBiase SJ, Kwok Y, Yovino S, et al. Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys 2004;60:1515-9. [Crossref] [PubMed]
- Adler JR, Jr., Gibbs IC, Puataweepong P, et al. Visual field preservation after multisession cyberknife radiosurgery for perioptic lesions. Neurosurgery 2006;59:244-54; discussion -54.
- Conti A, Pontoriero A, Siddi F, et al. Post-Treatment Edema after Meningioma Radiosurgery is a Predictable Complication. Cureus 2016;8:e605. [PubMed]
- Girvigian MR, Chen JC, Rahimian J, et al. Comparison of early complications for patients with convexity and parasagittal meningiomas treated with either stereotactic radiosurgery or fractionated stereotactic radiotherapy. Neurosurgery 2008;62:A19-27; discussion A-8.
- Marchetti M, Bianchi S, Pinzi V, et al. Multisession Radiosurgery for Sellar and Parasellar Benign Meningiomas: Long-term Tumor Growth Control and Visual Outcome. Neurosurgery 2016;78:638-46. [Crossref] [PubMed]
- Arvold ND, Niemierko A, Broussard GP, et al. Projected second tumor risk and dose to neurocognitive structures after proton versus photon radiotherapy for benign meningioma. Int J Radiat Oncol Biol Phys 2012;83:e495-500. [Crossref] [PubMed]
- Wenkel E, Thornton AF, Finkelstein D, et al. Benign meningioma: partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys 2000;48:1363-70. [Crossref] [PubMed]
- Slater JD, Loredo LN, Chung A, et al. Fractionated proton radiotherapy for benign cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys 2012;83:e633-7. [Crossref] [PubMed]
- Noël G, Bollet MA, Calugaru V, et al. Functional outcome of patients with benign meningioma treated by 3D conformal irradiation with a combination of photons and protons. Int J Radiat Oncol Biol Phys 2005;62:1412-22. [Crossref] [PubMed]
- Niranjan A, Kondziolka D, Lunsford LD. Neoplastic transformation after radiosurgery or radiotherapy: risk and realities. Otolaryngol Clin North Am 2009;42:717-29. [Crossref] [PubMed]
- Jääskeläinen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol 1986;26:461-9. [Crossref] [PubMed]
- Pollock BE, Link MJ, Stafford SL, et al. The Risk of Radiation-Induced Tumors or Malignant Transformation After Single-Fraction Intracranial Radiosurgery: Results Based on a 25-Year Experience. Int J Radiat Oncol Biol Phys 2017;97:919-23. [Crossref] [PubMed]
- Minniti G, Traish D, Ashley S, et al. Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma: update after an additional 10 years. J Clin Endocrinol Metab 2005;90:800-4. [Crossref] [PubMed]
- Breen P, Flickinger JC, Kondziolka D, et al. Radiotherapy for nonfunctional pituitary adenoma: analysis of long-term tumor control. J Neurosurg 1998;89:933-8. [Crossref] [PubMed]
- Gondi V, Hermann BP, Mehta MP, et al. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys 2013;85:348-54. [Crossref] [PubMed]
- Darzy KH. Radiation-induced hypopituitarism. Curr Opin Endocrinol Diabetes Obes 2013;20:342-53. [Crossref] [PubMed]
- Jenkinson MD, Javadpour M, Haylock BJ, et al. The ROAM/EORTC-1308 trial: Radiation versus Observation following surgical resection of Atypical Meningioma: study protocol for a randomised controlled trial. Trials 2015;16:519. [Crossref] [PubMed]


