Expert consensus on the nutritional therapy for patients with malignancies
1. Introduction
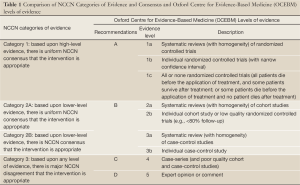
Continuous development of cancer treatment technologies and methods has extended the survival time of patients with malignant tumors, making these conditions more of a controllable and curable chronic diseases. Therefore, maintenance of the quality of life should be a major focus in the modern oncology. As an emerging interdisciplinary subject, nutritional oncology looks into the underlying mechanisms of malnutrition in malignant patients to identify the most appropriate methods for assessing the nutritional risks and status of cancer patients, seeking to improve the efficacy of anti-cancer therapy as well as the quality of life with nutritional therapy. What distinguish this from general nutriology is the abnormal metabolic state in patients with advanced and end-stage cancer as a result of the stress state and constant proliferation of tumor tissues in the tumor-bearing body. In addition, unlike surgery, radiotherapy, chemotherapy, molecular targeted therapy and other anti-cancer approaches, nutrition therapy does not kill tumor cells directly. Therefore, to establish the nutritional oncology with Chinese characteristics, oncologists and nutritionists should make joint efforts to continuously promote the research and development in this field (Table 1).
Full Table
Nutrition therapy has become an important component of the multidisciplinary treatment of patients with malignant tumors. To standardize nutritional therapy for patients at the perioperative phase and during chemotherapy or palliative period, and ensure reasonable, effective delivery of care, the Experts Committee on Nutritional Therapy for Cancer Patients of CSCO developed the expert consensus upon extensive consultation and public opinion research in accordance with the specific situation in China, and incorporating the latest guidelines for nutritional treatment by the European Society for Clinical Nutrition and Metabolism (formerly the European Society of Parenteral and Enteral Nutrition, ESPEN) and American Society for Parenteral and Enteral Nutrition (ASPEN). For the purpose of this consensus (1-6), the following terms are defined as follows:
- Nutritional therapy: the administration of a relatively comprehensive combination of nutrients, generally through the oral, enteral or parenteral routes, for metabolic conditioning;
- Enteral nutrition (EN): provision of nutrients through the digestive tract, including, based on different compositions, the delivery of macromolecule polymers (whole protein) and small molecule polymers (amino acids and short peptides);
- Parenteral nutrition (PN): intravenous delivery of nutrients, including amino acids, fats, carbohydrates, vitamins and minerals, to inhibit catabolism, promote anabolism, and maintain the functionality of structural proteins for patients in whom gastrointestinal uptake and utilization of nutrients is impossible;
- Malnutrition: an adverse event for both body functions and clinical outcomes resulting from deficient or excessive energy, protein and other nutrients;
- Nutritional insufficiency: usually referring to protein-energy malnutrition (PEM), a set of specific symptoms related to nutritional deficiencies in patients who have insufficient energy or protein intake, or malabsorption;
- Nutritional risk: the probability of negative impact on disease or surgery-related clinical outcomes (infection-related complications, length of stay, etc.) arising from the existing or potential nutritional and metabolic status;
- Nutritional risk screening: a fast, easy way used by clinical care providers to determine whether a further comprehensive nutritional assessment and a nutritional treatment plan are needed for a certain cancer patient;
- Nutritional assessment: a comprehensive examination and evaluation of the nutrition metabolism and body functions for a patient, used by nutritionists to take into account indications and possible side effects when developing nutrition treatment plans; and
- Cachexia: a complex syndrome found in cancer patients, characterized by chronic, progressive and unconscious weight loss, often accompanied by loss of appetite, satiety and fatigue, which can be either insensitive or partially sensitive to nutritional therapy.
2. Nutrition risk screening and assessment for tumor patients
Successful nutrition therapy relies first on an accurate assessment of the nutritional status of each cancer patient, identification of those eligible for the therapy by screening, and timely delivery of care. For objective evaluation of the therapeutic efficacy, follow-up evaluation will be needed during the treatment course for timely adjustment of the treatment plan.
The following concepts should be clarified for the purpose of assessing the nutritional status of patients with malignant cancer. First, malnutrition consists of both undernutrition and obesity (overweight), where undernutrition is determined by a body mass index (BMI) of <18.5 kg/m2 in combination with indicative clinical manifestations. Second, a nutritional risk describes the probability of an adverse effect on the clinical outcomes (such as infection-related complications, costs, and length of stay) of a patient due to his/her disease, surgery and nutritional factors, rather than the risk of occurrence of malnutrition (undernutrition). The nutritional risk can be interpreted in two ways: (I) patients with a higher risk are prone to adverse clinical outcomes; and (II) high-risk patients may benefit more from nutritional treatment.
Nutritional status assessment is completed in two steps: a preliminary screening, and then a comprehensive assessment. While the second step is a continuation of the former, they may not be confused with each other. The main purpose of the first step is to identify patients with existing malnutrition (undernutrition) or corresponding risks, particularly high-risk individuals who have not yet developed undernutrition, which should be completed at visit or upon admission to facilitate the formulation of nutrition treatment plans as clinically indicated. On the other hand, the second step, with a wider range of goals, is a comprehensive analysis of various nutritional parameters performed at any time when necessary to identify complications due to malnutrition (undernutrition), estimate nutritional requirements, develop nutrition treatment plans, and evaluate the therapeutic effect.
2.1 Nutrition risk screening
Screening approaches are developed to be simple and efficient with high sensitivity. Commonly used screening tools include: Subjective Globe Assessment (SGA), Mini Nutritional Assessment (MNA), Malnutrition Universal Screening Tools (MUST), Nutritional Risk Screening 2002 (NRS2002) (Table 2), and Patient-Generated Subjective Global Assessment (PG-SGA) (Table 3). SGA (7), an ASPEN-recommended tool for clinical nutritional status assessment published in 1987, incorporates a detailed medical history and physical assessment parameters that can be used to predict the incidence of complications. However, it is not capable of identifying mild undernutrition or reflecting the changes of acute nutritional status, and the correlation with clinical outcomes is not well supported by evidence. Therefore, this tool is more suitable for use by trained professionals, rather than serving as a routine nutrition screening tool in large hospitals. PG-SGA is a modified version of SGA that has been widely used for rough screening, the preferred method of nutritional screening in cancer patients recommended by the American Dietetic Association. MNA (8) is a fast, simple and easy-to-use tool published in 1999, which includes both nutrition screening and nutrition assessment, making it suitable for both patients with nutritional risks and undernourished hospitalized patients. The tool is useful for patients at the age of 65 or above and community populations. MUST (9), published by the Multidisciplinary Advisory Group on Malnutrition of the British Society for Parenteral and Enteral Nutrition in 2000, is mainly designed for screening protein-energy malnutrition and its risk, and is thus a useful tool for nutritional risk screening in different medical institutions, particularly communities.

Full Table

Full Table
Published in 2003 by the Danish Association of Parenteral and Enteral Nutrition (detailed in Table 2), NRS2002 (10) is an ESPEN-recommended tool for inpatient nutrition risk screening, which includes three main components: (I) Score of impaired nutritional status (0 to 3); (II) Score of disease severity (0 to 3); and (III) Age score, where one extra point will be assigned to this category for patients aged 70 years or older. The total score is 0 to 7 points. The analysis of 128 randomized clinical trials (RCT) on nutrition therapy versus clinical outcomes has suggested that, when the NRS score is three or above, effective therapy (significantly improved clinical outcomes) is demonstrated in most studies; when the NRS score is lower than three, the therapy is ineffective for most of them. The cut-off point for nutritional risks is thus set at three, meaning that a nutritional risk is present when the NRS score reaches three points or above and warrants individualized nutritional intervention plan based on the specific clinical conditions. Although patients with a NRS score lower than three are free of nutritional risks, a weekly screening is still needed during hospitalization (11).
Supported by the 128 RCTs, NRS2002 is an evidence-based, simple and easy-to-use tool that objectively reflects the nutritional risks of patients based on comprehensive analysis of the nutritional status, disease severity and age-related compounding factors to minimize subjective disturbance. Accordingly, the Parenteral and Enteral Nutrition Society of Chinese Medical Association has chosen and recommended NRS2002 as the screening tool to identify patients in need of nutritional intervention because it: (I) is oriented towards hospitalized patients; (II) is evidence-based; and (III) simple, easy-to-use (12). As verified by Liang et al., the adaptability of NRS2002 was 94.0% and 99.5%, respectively, for patients in a teaching hospital in China and the other in the United States (13). A study conducted by Yu et al. also demonstrated that NRS2002 could be used as the preferred tool for nutrition risk screening (14). Chen et al. conducted a feasibility study on NRS2002 for inpatient nutrition risk screening in China; as confirmed by their results, it is feasible to use NRS2002 for nutrition risk screening of Chinese hospitalized patients to identify those in need of nutritional intervention, based on the BMI values of the Chinese population (15).
However, NRS2002 is still associated with certain drawbacks. For example, body weight measurement is not possible for patients lying in bed, or will be inaccurate when edema or ascites is present. Unconscious patients who are unable to answer the questions are also ineligible for being assessed with this tool. Although serum albumin measurement may provide supplementary information, it can only be used in patient without significant liver and kidney dysfunction. In addition, NRS2002 may have limitations when applied to the special group of patients with malignant tumors. First, all subjects observed in the 128 RCTs were hospitalized patients, whereas the outpatient day treatment model has been gradually popular in clinical practice for cancer patients receiving radiotherapy and chemotherapy, making the use of this tool controversial. Second, the RCTs were conducted in almost exclusively general hospitals, mostly from the 1970s to 1990s, when the principles for malignant cancer treatment were considerably different from today’ s standardized multidisciplinary model, and the observation of specific clinical outcomes related to malignant cancer was not as accurate as it was supposed to be. Third, the classification of tumors into “tumors” and “hematological malignancies” in the disease severity section of NRS2002 remains controversial. With the two categories being assigned with one and two points, respectively, gastrointestinal tumors or head and neck cancer associated with a higher incidence of cachexia were not distinguished from breast cancer and other tumors that had a relatively better nutritional status. Meanwhile, standardized nomenclature of major abdominal surgeries has yet to be introduced in this tool.
Nevertheless, NRS2002 remains the most evidence-based tool for nutrition risk screening compared with its counterparts. In 2004, the Parenteral and Enteral Nutrition Society of Chinese Medical Association hosted the first inpatient nutrition risk screening with NRS2002 in tertiary Class A hospitals in large cities. According to the results of 15,098 hospitalized patients, NRS2002 is applicable for 99% or more Chinese hospitalized patients based on the normal BMI values of the Chinese people (16). They then conducted a prospective study of patients with malignant tumors admitted in large, medium and small hospitals in the eastern, central and western part of China from March 2005 to October 2008, finding that 40-41% of the subjects had nutrition risks that warranted a nutritional treatment plan based on their specific conditions. However, only 46% of those high-risk patients had received nutritional intervention. Malnutrition (undernutrition) and nutritional risks both increase along with age, indicating the need to emphasize nutrition therapy for elderly cancer patients. As the foregoing prospective study was carried out in the general wards of general hospitals, and did not involve specialized cancer centers or dedicated wards, or end-stage patients, it was unable to reflect the actual condition of nutrition risk screening for Chinese patients with malignant cancer. Therefore, in 2010, the Experts Committee on Nutritional Therapy for Cancer Patients of CSCO conducted a large-scale prospective observational study in cancer centers and specialized wards across the country, aiming to provide more evidence for the applicability of NRS2002 in malignant patients.
2.2 Further comprehensive nutrition assessment
After screening, patients with nutritious risks have to undergo the process of assessment before they are considered in need of medical nutrition therapy, which is conducted in combination with medical history review, physical examination, laboratory tests, anthropometry and a number of other indicators.
- Medical history: a patient’s acceptance of nutrition therapy is affected by previous tumors, past medical history, dietary surveys, drug history, social habits, lifestyle, health insurance, religious and cultural background, as well as economic situation;
- Physical examination: the depletion of adipose tissues and muscle tissues, presence of edema and ascites, and appearance of hair and nails, skin and oral mucosa are investigated to help evaluate the severity of energy and protein deficiency. Weight loss is not the only manifestation of malnutrition (undernutrition), as overnutrition and undernutrition can be simultaneously present in many patients, obscuring the differential diagnosis of malnutrition (undernutrition). The condition is often overlooked in obese patients;
- Laboratory tests: organ functions are investigated as an essential part in cancer treatment. Plasma proteins, blood urea, creatinine, plasma C-reactive protein (CRP), and immune function can be used as non-specific reference indicators;
- Body measurements: Dynamic weight monitoring is the most convenient and direct clinical indicator, but it is vulnerable to interference, such as fluid retention, coma, paralysis, edema, and huge tumor. In addition, the specific time and accurate result of the last weighing are often hard to trace for many patients. Other indicators include upper arm circumference (AC), triceps skinfold thickness (TSF), arm muscle circumference (AMC), reaction fat, and skeletal muscle reserve.
- Determination of body function and composition: changes in body function and composition can provide certain information for nutritional assessment. Nutrition therapy is an important link in the comprehensive treatment of malignant tumors, and the assessment of nutritional status should be conducted simultaneously with the assessment of the tumor, therapeutic effect, physical state and quality of life.
CT or MRI assessment of muscle mass was first introduced in the Definition and Classification of Cancer Cachexia: an International Consensus in 2010, as an extremely important component of the assessment system. It is not only one of the criteria for diagnosis, but also one of the goals of treatment, because the reduction of muscle mass is more critical than that of fat during weight loss, and low muscle mass is an independent predictor of mortality for patients with advanced tumors;
The efficacy of nutritional therapy should eventually be reflected in improved quality of life and higher tolerance of anti-tumor therapy. In terms of the former, efficacy monitoring can be used in a clinical study with focus on the impact of hospital stay, complications, adverse reactions, nutritional status, immune function, and organ function on the quality of life; for the latter, a rigorously designed, randomized, controlled trial or retrospective cohort study can be performed to observe the overall survival for comparing the long-term efficacy with nutritional therapy of different approaches, time and formulas, identifying the need of combined nutrition support in anti-tumor therapy, and determining the impact of combined therapy on the long-term survival, with the aim to establish the most scientific nutrition therapy model.
2.3 Recommendation
- Upon a definite diagnosis, patients with malignant tumors should be subject to nutritional risk screening immediately (Level 1).
- At present, PG-SGA and NRS2002 are the most widely used tools for nutrition risk screening of patients with malignancies (Level 1).
- A nutritional risk is determined when the NRS score reaches three or above, where an individualized nutrition plan should be developed according to the patient’s clinical conditions and nutritional intervention be administered (Level 2A).
- Although patients with a NRS score lower than three are free of nutritional risks, a weekly screening is still needed during hospitalization (Level 2A).
- Medical history, physical examination and laboratory tests are helpful in understanding the cause and severity of malnutrition in patients with malignant tumors, facilitating a comprehensive nutrition assessment (Level 2A).
- Nutrition risk screening and comprehensive nutrition assessment should be conducted simultaneously in combination with imaging evaluation of anti-tumor efficacy to provide an all-round assessment of the benefits from anti-cancer therapy(Level 2A) .
3 Nutritional therapy for non-end-stage cancer patients undergoing surgery
Surgical treatment for non-end-stage cancer patients includes radical surgery and palliative surgery, with an attempt to extend survival and improve quality of life. Therefore, nutritional therapy for these patients should aim at improving their tolerance to surgery, reducing the incidence of surgical complications, and lower the mortality. Severe malnutrition (under-nutrition) is an important factor affecting the clinical outcome of surgical patients; furthermore, inappropriate nutrition therapy also harms patients. Therefore, it is essential to provide appropriate nutrition therapy for preoperative patients.
3.1 Goal and effectiveness of nutrition therapy for non-end-stage cancer patients undergoing surgery
For patients undergoing major surgery with moderate or severe malnutrition (under-nutrition), nutritional therapy initiated 10-14 days before surgery can reduce the incidence of surgical complications (17). Among 32 RCTs, 24 have showed that enteral nutrition (EN) reduced postoperative infection-related complications, hospital stay, and hospitalization costs, while the other 8 RCTs showed negative results. For gastrointestinal cancer patients with under-nutrition, early EN reduced the incidence of postoperative infection (compared with total parenteral nutrition), but showed no such effect on patients with normal nutrition status (18).
The conventional 10-12-hour fasting before surgery is not conducive to the post-operative rehabilitation because it causes the body to prematurely enter into a catabolic status. Evidence has shown that liquid food intake 2-3 hours before surgery did not increase the risk of regurgitation and aspiration; therefore, the societies of anesthesiologists in many countries have rescheduled the time of preoperative fasting to 6 hours for patients undergoing elective surgery, while preoperative water deprivation only 2 hours (19). For patients undergoing major surgery, carbohydrate load (800 mL the night before and 400 mL two hours before surgery) did not increase the risk of aspiration (19). For patients undergoing colorectal surgery, hypotonic carbohydrate intake before surgery could alleviate postoperative insulin resistance (20), reduce skeletal muscle decomposition, and improve the tolerance; particularly, these patients have better postoperative muscle strength (21). For those who are not able to take oral carbohydrate preoperatively, intravenous glucose at a rate of the 5 mg/kg/min can be provided, so as to reduce insulin resistance, decrease protein consumption, and protect the myocardium (22).
Compared with parenteral nutrition (PN), EN is more accorded with physiological requirement and more conducive to maintaining the structure and functional integrity of the intestinal mucosal cells, with fewer complications; furthermore, it is more affordable. Therefore, EN should be preferred as long as some of the gastrointestinal digestion and absorption functions still exist. Some patients are not able to tolerate EN due to the abnormal anatomic or functional reasons of gastrointestinal tract, or EN alone is far from being sufficient to meet the metabolic demands; in these patients, PN can be a necessary approach for supporting metabolism. However, once the intestinal tract regains its normal functions, EN should be applied.
Early feeding or EN is also beneficial for patients undergoing colorectal surgery. It has been suggested that early postoperative feeding or EN (including liquid food intake within 1-2 days after surgery) does not affect colorectal anastomotic healing (23). However, it is unclear whether early intake of nutrients through the digestive tract has any impact on patients undergoing major gastrointestinal surgery in the upper abdomen. The current expert consensus is that the start time and the dose of the early postoperative feeding or EN should be decided according to the gastrointestinal functions and the tolerability of the patients.
Immediate or gradual withdraw of PN shows no difference in affecting blood glucose level (24). So far, no evidence supports that the regeneration of tumor cells is more vigorous than that of other somatic cells; meanwhile, no research suggests that such regeneration would cause harmful clinical outcomes. Therefore, it is not justifiable to giving up PN due to concerns about its supportive effect on tumor growth (25). For patients who are not able to obtain adequate nutrition from normal diets after discharge, EN supplements are beneficial to improve their nutrition status and reduce complications (26).
3.2 Indications of nutrition therapy for non-end-stage cancer patients undergoing surgery
Multivariate analysis showed that undernutrition is an independent risk factor for postoperative complication, and is associated with higher mortality, longer hospital stay and high hospitalization costs (27). For patients undergoing major surgery with moderate or severe malnutrition (under-nutrition), nutritional therapy initiated 10-14 days before surgery can reduce the incidence of surgical complications (17).
For mildly undernourished patients, however, pre-operative PN is useless, and may even increase the risks of infectious complications (28). Also, patients without malnutrition or can obtain sufficient enteral nutrition within 7 days after surgery can not benefit from PN (29). Both the infection rates and the hospitalization stay are both lower in patients receiving EN than those receiving PN; however, the contraindications of EN including intestinal obstruction, hemodynamic instability, and intestinal ischemia must be ruled out. Although few case-control studies have explored the role of the combined application of EN and PN, it is commonly agreed that EN + PN can be considered for patients with the indications of nutritional therapy but their energy demands can not be met by EN alone (<60% of caloric requirements).
3.3 Method and special ingredients of nutritional therapy
Preoperative under-nutrition is more common in patients with head and neck malignancies. The high risk of postoperative infection and high incidences of postoperative anastomotic edema, obstruction and delayed gastric emptying often lead to delayed oral feeding; therefore, tube feeding nutrition should be considered, which can be carried out within 24 hours after surgery (30). It is safe to place feeding tube by percutaneous jejunostomy in patients undergoing major abdominal surgery; meanwhile, it is also safe to place nasojejunal feeding tube for patients undergoing pancreaticoduodenectomy. For patients undergoing proximal gastrointestinal anastomosis, EN can be provided through feeding tube with the top located in the distal end of anastomotic. For patients undergoing long-term (>4 weeks) tube feeding nutrition (e.g., patients with severe head and neck trauma), feeding tube can be placed by percutaneous endoscopic gastrostomy if no abdominal surgery is required. Considering the intestinal tolerance, it is usually feasible to carry out tube feeding nutrition at a low drip rate (e.g., l0-20 mL/h); thus, it may take 5 to 7 days to achieve a sufficient amount of nutrition intake. For perioperative patients receiving nutrition therapy, the nutritional status should be routinely re-evaluated during hospitalization; if necessary, nutritional therapy should be continued after discharge.
The energy and protein demands of cancer patients do not differ from those of healthy subjects; thus, the estimated energy demand of a bedridden patient is about 20-25 kcal/kg per day, while that of an ambulatory patient is 25-30 kcal/kg per day (31). If severe dysfunction of heart, liver, kidney, and/or intestines occurs, appropriate nutritional therapy should be provided (32). EN therapy with the formula of standard macromolecule polymer (whole protein) is applicable for most patients. A meta-analysis showed that the peri-operative application of EN containing immunomodulatory component (arginine, omega-3 fatty acids and nucleotides) in patients undergoing major surgery (e.g., laryngectomy or pharyngeal part resection) for neck tumors or those undergoing major surgery (e.g., esophageal resection, gastrectomy, or pancreaticoduodenectomy) for abdominal tumors could reduce complications and shorten hospital stay (33). However, for critically ill patients with systemic infections, EN containing arginine may increase the mortality (34). For postoperative patients without malnutrition undergoing oral feeding or EN, no sufficient evidence shows that intravenous supplement of vitamin and trace elements is feasible; however, for those with malnutrition and EN is not feasible, daily supplement of vitamins and trace elements is mandatory (35). Research has shown that insulin may promote synthesis metabolism in tumor patients; therefore, it may be beneficial for patients with weight loss to receive subcutaneous insulin and proper nutrition therapy (36).
3.4 Recommendation
- A routine 12-hour preoperative fasting is not recommended for patients undergoing elective surgery without delayed gastric emptying. For surgical patients without special risk for aspiration or delayed gastric emptying, only water deprivation two hours before anesthesia and fasting 6 hours before surgery is recommended. Intravenous carbohydrates can be provided for patients who are unable to eat before surgery (Level 1).
- The nutritional intake should not be interrupted for the majority of patients. Normal food intake or EN should be initiated early after surgery. Most colectomy patients can orally take liquid food (including water) a few hours after surgery (Level 1).
- For patients with a risk of severe under nutrition, nutritional therapy should be provided 10 to 14 days before major surgery. For perioperative severe undernourished patients who are unable to obtain sufficient nutrition by oral feeding or EN for 5-l0 consecutive days, PN therapy should be provided (Level 1).
- Tube feeding should be provided for patients who can not receive early oral nutritional therapy, especially for those who has undergone major surgeries on the head, neck, or gastrointestinal tract, experienced severe trauma, or has obvious under-nutrition. Smaller Jejunal fistula or nasojejunal tube is recommended for all patients who has received abdominal surgery and required tube feeding nutrition (Level 1).
- For patients undergoing major neck surgery and abdominal surgery, the perioperative EN containing immunomodulatory components (arginine, omega-3 fatty acids, and nucleotides) can be considered (Level 1).
4. Nutritional therapy for non-end-stage cancer patients on chemotherapy
The “non-end-stage cancer patients on chemotherapy” refers to patients who have indications for chemotherapy and expected survival time over three months. For these patients, clinicians will take a series of aggressive anti-tumor treatment to control disease progression or prolong the survival.
Unlike surgery and other localized treatment, chemotherapy is a systemic treatment to kill tumor cells and thus often causes significant toxicity, especially gastrointestinal reactions such as nausea and vomiting, abdominal pain, diarrhea and gastrointestinal mucosal injury, that will seriously impair the patient’s appetite or affect their eating, resulted in an enhanced malnutrition due to abnormalities in the metabolism of cancer patients. Second, poor nutrition will reduce patient’s tolerance to chemotherapy (37) and affect the level of neutrophils (38), resulting in failure of a full course of chemotherapy (or, the early termination of chemotherapy treatment), thus affecting the therapeutic effect of anti-tumor therapy. Therefore, clinicians should pay special attention to the risk of malnutrition induced by chemotherapy in cancer patients and make a serious assessment of the patients’ nutrition status as well as an early response to malnutrition following chemotherapy, so as to maintain a good nutrient levels and provide appropriate metabolic environment for chemotherapy.
4.1 Objectives and outcomes of nutritional therapy for non-end-stage cancer patients on chemotherapy
The primary goals of nutritional therapy for non-end-stage cancer patients on chemotherapy are: (I) to prevent and correct malnutrition or cachexia; (II) to improve the tolerance and compliance of patients to chemotherapy; (III) to control the side effects of cancer chemotherapy; (IV) to improve the quality of life.
For patients on conventional chemotherapy, nutritional therapy can improve their life quality (39-41). A report from Germany published in 2006 investigated 152 patients who received combined treatment with radiation and chemotherapy for gastrointestinal, pancreatic, ovarian and/or breast cancer. PN+EN remarkably improved the patients’ appetite and quality of life compared with EN alone (40). In 2009, a randomized controlled trial (RCT) of 82 patients with advanced colorectal cancer drew a similar conclusion (42).
With an attempt to increase the energy intake during chemotherapy, nutritional therapy is beneficial for cancer patients on chemotherapy (40,43). Another study, however, found that of nutritional therapy in the maintenance of patient’s body weight was limited (44). As shown by an RCT on breast cancer patients on chemotherapy, the serum levels of transferrin and albumin did not remarkably increase after EN support (45).
In terms of clinical outcomes, for chemotherapy patients with gastrointestinal tumors or non-gastrointestinal tumors, the impact of nutritional therapy on the outcome is very limited. Over the past 30 years since 1970s, several small RCTs (maximum sample size: 192 cases) have been performed to investigate the impact of nutritional therapy on the clinical outcomes of patients undergoing chemotherapy against malignancies of gastrointestinal tract, lung, breast, malignant lymphoma, and testicular cancer. They focused on the toxicities of conventional chemotherapy, the patient’s response to chemotherapy, and their survival time. However, no convincing evidence has demonstrated that the routine application of nutritional therapy can reduce chemotherapy toxicity (46,47), promote patient’s response to chemotherapy(46,48), or extend survival (44,49). Nevertheless, it has also been proposed that parenteral nutrition (PN) plus enteral nutrition (EN) is more effective than EN alone in lowering the chemotherapy-associated toxicity (42). It is notable that these conclusions were based on small-sample RCTs, in which the nutrition status of patients was normal or only mildly impaired. Moreover, the conclusions from some RCTs were less reliable due to the diversities of tumor types, chemotherapy protocols, and nutrition programs. More importantly, a meta-analysis on cancer chemotherapy patients under 21 years published in July 2010 revealed that the efficacy of PE was not superior to EN in patients with good nutrition status (50).
In 2009, the Comparing Parenteral Nutrition vs. Best Supportive Nutritional Care in Patients with Pancreatic Cancer (PANUSCO) (51) was launched in Germany. Targeting at patients with pancreatic cancer, this RCT was designed to investigate the effect of nutritional therapy on the clinical outcomes and life quality of patients after the application of a uniform chemotherapy program. Although its final results have not finally released, this study may address the impacts of different chemotherapy programs and bring more useful information for guiding nutritional therapy in patients on chemotherapy.
4.2 Indications of nutritional therapy for non-end-stage cancer patients on chemotherapy
It is still controversial whether nutritional therapy should be given, subject to medical ethics, to patients on chemotherapy in following conditions: (I) the daily energy intake is lower than 60% of daily energy consumption for more than 10 days; (II) patients are expected to be fasting for 7 days or more; and (III) or patients suffer from weight loss. Currently no evidence from large-sample evidence-based studies has been available (1,47). PN, however, is recommended for patients who have developed mucositis or severe radiation enteritis (52).
4.3 Method, energy, and special ingredients of nutritional therapy
Patients on EN has the same risk of infections as those without nutritional therapy (53), while PN can increase the risk of infections (54). No evidence has shown that nutritional therapy can promote tumor growth; thus, it is not necessary to consider this issue when make a decision for nutritional therapy.
Polyunsaturated fatty acids (EPA) from fish oil have shown active efficacies in animal tumor models (55). In vitro studies have demonstrated the inhibitory effect of EPA on tumor cell growth (56). However, findings from clinical studies with large sample sizes were still controversial. Dewey et al. reviewed EPA studies and found oral EPA was not beneficial for patients with cachexia (57). Nevertheless, it is also notable that, inside this review, the dose of EPA was insufficient in at least two studies and therefore could not achieve the goal of the treatment. Another three studies had limitations such as the short administration duration and the misleading data from patients with gastrointestinal cancer. In 2004, a study on 421 cancer patients undergoing chemo-radiotherapy showed that supplementation with EPA could neither improve the quality of life, nor increase the patient’s body weight (58). However, recently a meta-analysis (59) on 12 RCTs on patients with prostate cancer showed that although EPA intake did not reduce the incidence of prostate cancer, it did reduce the mortality. According to Gogos et al., EPA extended the survival of patients (60).
According to the report published in November 2010 by an American research group, the nutritional formula supplement with glutamine can play a certain role in inhibiting systemic metastasis in mice tumor models (61). Moreover, the results of another study using mice colon carcinoma models demonstrated that combined treatment with glutamine and n-3 unsaturated fatty acids did not inhibit tumor growth, but promoted an increase in body weight of mice undergoing chemotherapy with CPT-11 and 5-Fu, improved their appetite, elevated level of leukocytes, and significantly enhanced the tolerance of animals to chemotherapy (59). However, the similar effects in human subjects remain to be validated in future. A recent Phase III clinical trial in patients with colorectal cancer in the United States (62) showed that supplementation with complex vitamins during or after chemotherapy did not affect the recurrence rate and survival time of stage III colorectal cancer patients.
4.4 Recommendation
- Although nutritional therapy can improve the quality of life of patients on chemotherapy and increase their appetite, the currently available studies show that nutritional therapy has no apparent effect on the blood biochemical parameters and clinical outcomes in these patients. Therefore, routine nutritional therapy is not recommended for patients who are on chemotherapy but not undernourished (Level 1).
- Nutritional therapy should be initiated for the following patients to fill the gap between actual intake and theoretical intake: (I) the daily energy intake is lower than 60% of daily energy consumption for more than 10 days; (II) patients are expected to be fasting for 7 days or more; and (III) or patients suffer from weight loss (Level 2A). EN is preferred to lower the risk of infections (Level 2A). A short-term PE program may be considered for patients with chemotherapy-induced gastrointestinal mucosal injury (Level 2A).
- Nutritional therapy for tumor patients should use standard formula (Level 2A).
- Intake of compound vitamins during themotherapy does not affect the relapse rate and survival in patints with phase III colorectal cancer (Level 2A).
- No evidence has shown that nutrition support may also nourish tumors. Therefore, it should be applied when clinical indications exist (Level 2A).
5. Nutritional therapy for non-end-stage cancer patients on radiotherapy
Radiotherapy is one of main treatment options for malignant tumors and about 70% of patients with malignant tumor may receive radiotherapy throughout the course of the disease. Major causes of malnutrition in cancer patients are: (I) tumor-associated abnormal metabolism; (II) malnutrition caused by dysfunction of the organs where tumor is growing; and (III) toxic effects of anti-cancer treatment. Radiotherapy works by killing tumor cells through a mechanism underlying direct and indirect DNA damage that is caused by X-ray-mediated DNA single or double-strand breaks. However, radiotherapy not only kills cancer cells, but also brings severe toxic effects to the adjacent normal tissues, particularly when combined with chemotherapy. The toxicity of radiotherapy or chemotherapy can be divided into systemic or local reactions. The systemic reactions (e.g., fatigue and anorexia) are usually nonspecific. On the contrary, the local reactions are responses of local normal organs that are exposed to X-rays during the treatment; for example, oral mucosa injury can occur during radiotherapy for head and neck cancer, whereas radiation-induced esophageal injury may develop during radiotherapy for thoracic tumor.
Malnutrition in patients on radiotherapy mainly occurs in the organs within the field of radiation. The common causes of malnutrition in patients on radiotherapy are: (I) adverse reactions such as oral mucositis, pharyngeal pain, bad appetite, and loss of taste during radiotherapy for head and neck tumor can lead to an insufficient intake of nutrition; (II) insufficient nutrition intake caused by radiation esophagitis after radiotherapy for chest tumors; and (III) damages of the mucosa in the gastrointestinal tracts after abdominal tumor radiotherapy can cause poor appetite, nausea, vomiting, and diarrhea, which ultimately result in insufficient intake or poor absorption of nutrients. These adverse reactions appear about 3 or 4 weeks after radiotherapy and can last more than 2 weeks after the completion of radiotherapy (63). Meanwhile, the tumor itself can also affect the appetite or food intake of cancer patients, leading to malnutrition, lower tolerance to treatment, and even a suspension or an early termination of therapy; thus, the overall treatment efficacy will also be lowered. For patients with tumors of head and neck or gastrointestinal tract, dietary guidance and oral nutritional supplements (ONS) may prevent the weight loss or the early termination of therapy during radiotherapy.
The aims of nutritional therapy for non-end-stage cancer patients on radiotherapy are: (I) assess, prevent and treat malnutrition/cachexia; (II) improve the tolerance and compliance of patients to the anti-cancer therapy; (III) control the certain adverse reactions of anti-tumor therapy; and (IV) improve the quality of life (64,65).
5.1 Goal and effectiveness of nutritional therapy for non-end-stage cancer patients on radiotherapy
It has been widely recognized that mucositis during radiotherapy and chemotherapy in patients with head and neck cancer or esophageal cancer may lead to weight loss (66-69), which can be prevented by nutritional support. According to Isenring, for an ambulatory patient on radiotherapy for his/her head and neck or gastrointestinal cancer, prompt nutrition intervention can effectively reduce their weight loss, prevent deterioration of their nutritional status, and improve their quality of life (70). Bozzetti et al. (71) also found that in patients with esophageal cancer after chemotherapy or radiotherapy, home-based EN program could prevent further deterioration of patient’s nutritional status that was caused by poor nutrition due to swallowing difficulty. Several prospective (71,72) and retrospective studies (68,73-75) also confirmed that, compared with routine diets, oral nutritional therapy or nasogastric intubation feeding can effectively reduce the weight loss.
5.2 Indications of nutritional therapy for non-end-stage cancer patients on radiotherapy
Fewer studies have reported the feasibility of routine nutritional therapy during radiotherapy (76-79); notably, one study on upper digestive tract tumor and another study on head and neck cancer have shown that patients who had received EN prior to radiotherapy had lower risks of weight loss and early termination of therapy (76,80). Another two studies on head and neck cancer showed that PN and EN before radiotherapy did not alleviate weight loss (77) and the prognoses were poor (78). In 2001, Koretz reviewed randomized clinical trials on nutritional intervention during chemo- and radio-therapies and found that, in the absence of malnutrition, PE is not beneficial and even may harm the body (81). However, PE may benefit patients with malnutrition or serious iatrogenic gastrointestinal complications; unfortunately, it is not ethical to carry out randomized comparison study on this condition (82).
The role of PN in the treatment of severe mucositis or severe acute radiation enteritis has been widely accepted (83). According to severity of post-radiation oral mucosal reactions, nutritional therapy should be actively applied for patients with a rating at level 3 or above. Long-term parenteral nutrition is also widely recognized for the patients with subacute or chronic radiation enteritis (84,85). In 2006 a German study investigated 152 cancer patients receiving combined treatment with radiation and chemotherapy for tumors including gastrointestinal, pancreatic, ovarian and breast cancer and found that the patient’s appetite and quality of life functions scores were better with additional parenteral nutrition, as compared with enteral nutrition alone (86).
5.3 Modalities of nutrition therapy
EN may be applied via nasogastric intubation feeding or percutaneous placement of gastric feeding tubes. Gastrointestinal intubation feeding can be administered for those who have swallowing difficulty caused by obstructive head and neck cancer or esophageal cancer as well as those who have swallowing difficulty due to local severe mucositis, such as laryngeal or esophageal cancer patients with radiotherapy and chemotherapy. EN can maintain quality of life (87), prevent treatment interruptions (67,88), and reduce the frequency of hospital re-admission (67,71,72).
5.4 Recommendation
- Nutritional assessment for patients with radiotherapy should be performed when they have a diagnosis of cancer or are admitted to hospital, in particular before and during radiotherapy. Update of the assessments is required in each sequential follow-up, so that the patients can be treated with early nutrition therapy and nutrition intervention prior to occurrence of systemic nutritional deficiencies (Level 2B).
- Daily consumption of a patient on radiotherapy is similar to healthy people. In general, a patient on radiotherapy needs KPS60 or above; thus the estimated daily intake is 25-30 kcal/kg/day for patients on radiotherapy (Level 2B).
- The purposes of PN for patients undergoing radiotherapy are to achieve an effective treatment of anti-cancer therapy by following approaches: prevent and treat malnutrition/cachexia; improve the tolerance and compliance of patients to radiotherapy; control or reduce the side effects of radiotherapy; and improve the quality of life (64,65) (Level 2B).
- PN is not necessary, or even harmful, for patients without gastrointestinal dysfunction (Level 1).
- Options of nutrition therapy: EN is preferred to reduce the risk of infection (Level 2a); EN should be given via tubes to the patients with swallowing difficulty caused by obstructive head and neck cancer or esophageal cancer (Level 2b); PN is recommended for patients who can not tolerate EN but in need of nutritional therapy, such as those who have severe mucositis after radiotherapy or severe radiation enteritis.
- Routine use of PN is not recommended for patients on radiotherapy who have neither status of malnutrition nor risk of malnutrition (Level 1).
6. Nutritional therapy for end-stage cancer patients
End-stage cancer patients are those who are no longer responsive to conventional anti-cancer treatment, such as surgery, radiotherapy, chemotherapy and molecularly targeted drug treatment. In general, the expected survival of these patients is less than three months.
Severe cachexia is often observed in patients with end-stage malignant tumors. A complex syndrome in cancer patients, cachexia is characterized by chronic and unknowingly progressive weight loss, often accompanied by anorexia, satiety and fatigue performance, which is unresponsive or partially responsive to nutritional therapy. There are generally two causes of cachexia: reduced nutritional intake, which may be a result of direct violation of the gastrointestinal tract by the tumor or digestive disorders induced through cytokines and relevant appetite inhibitors; and an abnormal metabolic state due to activation of proinflammatory cytokines, including cytokines generated in the body in response to tumor tissues, catabolic hormones and small regulatory peptides, and lipid mobilizing factor (LMF) and proteolytic inducible factor (PIF) produced by tumor tissues. These cytokines will enhance catabolism of the body via certain signaling pathways, and systemic inflammatory response can weaken appetite and lead to weight loss.
In the newly published Definition and Classification of Cancer Cachexia: an International Consensus, Kenneth Fearon first introduced his three-stage classification system for cachexia diagnosis, including: pre-cachexia stage, identified by weight loss ≤5% with anorexia or reduced glucose tolerance; cachexia stage, identified by weight loss >5% or >2% for those with a baseline BMI of <20 or with sarcopenia within six months; and refractory cachexia stage, when expected survival is less than three months, PS scores are low and the patient is unresponsive to anti-tumor treatment.
In principle, treatment for end-stage patients is aimed to maintain the quality of life and relieve symptoms, with the former being the most important component in the assessment of nutritional therapy.
6.1 Indications for end-stage nutritional therapy
Nutritional therapy for end-stage patients is not only a medical issue but also related to ethics and the preference of patients and their families. Although nutritional therapy can improve the quality of life for end-stage patients with malignancies, its ability of prolonging survival remains controversial. It has been reported that nutritional therapy alone can neither maintain the fat-free body weight nor improve the mean survival time and long-term survival for patients with severe protein-energy undernutrition and cachexia (89,90). In Asian countries, however, many end-stage cancer patients are still receiving the therapy without a hope of prolonging survival. Retrospective studies in Japan and Korea have shown that total parenteral nutrition and intravenous albumin is prescribed for a high proportion of end-stage cancer patients even within one month before death. In view of inadequate high Level evidence for decision-making in this regard, clinicians should base their decision of whether or not to administer nutritional therapy on careful risk-benefit assessment of the therapy for each patient following the indications based on clinical evidence and theory of social ethics, as well as respect for the willingness of patients and justifiable allocation of limited health care resources (91).
The principles of treatment for end-stage patients include reducing tumor burden, conditioning of gastrointestinal function, prescribing nutrients and energy supplement, and delaying the progression of cachexia so as to improve the quality of life.
Nutritional therapy is not recommended in patients near the end of life, as most of them need only a very small amount of food and water to reduce hunger and thirst, and prevent mental confusion due to dehydration. In this case, excessive nutritional therapy will instead increase their metabolic burden, thus affecting the quality of life (92). Systematic nutritional therapy is generally contraindicated in patients with unstable vital signs and/or multi-organ failure.
Apart from that, combination treatment with effective anticancer drugs, such as time-dependent chemotherapy and molecularly targeted therapy, is recommended for end-stage patients. Proactive nutritional therapy provides an opportunity for chemotherapy and molecular targeted therapy, as well as unresponsive patients. The combination of both is now believed to be helpful in improving the quality of life and survival (93).
6.2 Method, energy, and special ingredients of nutritional therapy
The treatment plan shall be developed based on the systemic nutritional status and gastrointestinal functions of patients. While the decision of enteral or parenteral nutrition should be based on specific conditions, close monitoring of fluid volume, edema or dehydration symptoms and signs, and blood electrolyte levels must be guaranteed in either case, in conjunction with timely adjustment and administration of doses. For patients with stable vital signs who are willing or have agreed to accept nutritional therapy, the treatment can be prescribed with enteral nutrition preferred for those with a functional digestive tract (90). Parenteral nutrition is used for patients experiencing loss of gastrointestinal function, but will be terminated immediately upon recovery of their bowel function, or when enteral nutritional therapy suffices for the energy and nutrient requirements. Enteral and parenteral nutrition is contraindicated in the case of hemodynamic instability. Parenteral routes are prohibited in patients with end-stage liver and kidney failure and severe biliary obstruction.
Nutritional therapy is provided for end-stage patients with the aim to maintain weight instead of gaining it, as excessive nutritional supply may increase organ load, and thus the total energy intake and proportion of heat-producing nutrient must be taken into consideration. Low calorie intake is helpful in reducing infectious complications and cost.
Glucocorticoid and megestrol acetate have been conclusively associated with increased appetite (94). Metabolic regulators can be used to reverse the abnormal metabolism in patients with cachexia metabolism, when necessary. These drugs include eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and a non-steroidal anti-inflammatory agent, thalidomide (95-98).
6.3 Management of complications
End-stage patients are prone to metabolic complications due to underlying electrolyte disorders and metabolic abnormalities (99):
- Glucose metabolism disorders: hyperosmolar hyperglycemic nonketotic coma is the major manifestation, which can be prevented by increased dosage of exogenous insulin and reduced infusion of exogenous glucose;
- Metabolic acidosis: decreased blood pH can be observed in cancer patients in the event of reduced use of sugar, elevated serum lactate and resultant decreased blood pH as a result of anaerobic glycolysis in tumor tissues, and the presence of titratable acids, such as 50% glucose and cationic amino acids, in the nutrient solution. This can be prevented with small doses of baking soda and reduced sugar infusion;
- Potassium disorders: this is most common when prescription is improper or bottle infusion is used. Hypokalemia is likely to occur in nutritional therapy as it promotes anabolism, where a large amount of potassium ions are transferred into cells during sugar infusion. Serum potassium concentration shall be monitored and potassium administered when needed;
- Fat overload: hyperlipidemia, organ dysfunction, hemolysis, unconsciousness and even coma may occur when the dosage and infusion rate of fat emulsion exceeds the ability of fat clearance, though this will subside upon termination of the infusion;
- Hyperammonemia: this is caused by excessively fast infusion of amino acids and reduced infusion of arginine, which can be prevented by slower infusion of amino acids and the addition of arginine solutions;
- Infections: long-term parenteral nutrition may lead to intestinal mucosal atrophy, intestinal dysfunction and bacteria translocation that result in intestinal-borne infections or catheter infections. Shortened parenteral nutrition and replacement with enteral nutrition as soon as possible will be helpful for prevention of these conditions.
6.4 Recommendation
- Nutritional therapy can improve the quality of life of end-stage cancer patients (Level 2A);
- Nutritional therapy alone can neither maintain the fat-free body weight nor improve the mean survival time and long-term survival for patients with severe protein-energy undernutrition and cachexia (Level 2A);
- Most patients near the end of life need only a very small amount of food and water to reduce hunger and thirst the end of life, while excessive nutritional therapy will instead increase their metabolic burden, thus affecting the quality of life (Level 2A);
- High-energy nutritional therapy is not recommended for patients with end-stage malignancies to achieve positive nitrogen balance or nitrogen balance (Level 2A);
- Proactive nutritional therapy provides a timeframe and opportunity for anti-tumor therapy, and the combination of both is helpful in improving the quality of life and survival (Level 2A);
- Formulation of required nutrients should be based on an individualized assessment of the disease status, body weight and body composition, as well as changes in physiological functions (Level 2A);
- Glucocorticoid and megestrol acetate are conclusively effective in increasing appetite (Level 1);
- While the decision of enteral or parenteral nutrition should be based on specific conditions, close monitoring of fluid volume, edema or dehydration symptoms and signs, and blood electrolyte levels must be guaranteed in either case, in conjunction with timely adjustment and administration of doses (Level 1).
Acknowledgements
Members of Experts Committee on Nutritional Therapy for Cancer Patients of CSCO (names in alphabet order)
CAI San-jun, CHEN Jin-fei, CHEN Wei, FAN Yun, JI Jia-fu, Jiang Zhi-wei, HUANG Jing, LI Su-yi, LIANG Hou-jie, LIN Feng, LU Jian-wei, MA Dong, PAN Hong-ming, SONG Chun, TAO Min, WANG Lin, WANG Zhe-hai, WU Guo-hao, XU Ye, YIN Yong-mei, YU Zhen, YU Zhuang, ZHANG Feng-chun, ZHANG Tao, ZHANG Shu, ZHANG Xiao-tian, ZHANG Zhen
Contributors
PAN Hong-ming, CAI San-jun, JIANG Zhi-wei, JI Jia-fu, LIANG Hou-jie, LIN Feng, ZHANG Zhen, ZHANG Xiao-wang, LI Su-yi
Revised by
PAN Hong-ming, CAI San-jun, CAO Wei-xin, WU Guo-hao, QIN Shu-kui, SUN Yan
If you have any comments on this document, please email us at: chenweizju@hotmail.com or zhangyi@eddingpharm.com.
Disclosure: The authors declare no conflict of interest.
References
- August DA, Huhmann MB, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr 2009;33:472-500. [PubMed]
- Bozzetti F, Arends J, Lundholm K, et al. ESPEN Guidelines on Parenteral Nutrition: non-surgical oncology. Clin Nutr 2009;28:445-54. [PubMed]
- Braga M, Ljungqvist O, Soeters P, et al. ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr 2009;28:378-86. [PubMed]
- Weimann A, Braga M, Harsanyi L, et al. ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr 2006;25:224-44. [PubMed]
- Arends J, Bodoky G, Bozzetti F, et al. ESPEN Guidelines on Enteral Nutrition: Non-surgical oncology. Clin Nutr 2006;25:245-59. [PubMed]
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [PubMed]
- Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr 1987;11:8-13. [PubMed]
- Guigoz Y. The Mini Nutritional Assessment (MNA) review of the literature--What does it tell us? J Nutr Health Aging 2006;10:466-85; discussion 485-7. [PubMed]
- Stratton RJ, King CL, Stroud MA, et al. ‘Malnutrition Universal Screening Tool’ predicts mortality and length of hospital stay in acutely ill elderly. Br J Nutr 2006;95:325-30. [PubMed]
- Kondrup J, Rasmussen HH, Hamberg O, et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003;22:321-36. [PubMed]
- Kondrup J, Allison SP, Elia M, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003;22:415-21. [PubMed]
- Chinese Medical Association. Guidelines on Clinical Diagnosis and Treatment: Parenteral & Enteral Nutrition. Beijing: People’ s Medical Publishing House, 2006:15-9.
- Liang X, Jiang ZM, Nolan MT, et al. Comparative survey on nutritional risk and nutritional support between Beijing and Baltimore teaching hospitals. Nutrition 2008;24:969-76. [PubMed]
- Yu K, Xia Y, Wang MZ, et al. Comparison of Nutritional Risk Screening and Subjective Globe Assessment for Nutritional Screening in Inpatients with Non-operative Pulmonary Carcinoma. Chin J Clin Nutr 2008;16:349-51.
- Chen W, Jiang ZM, Zhang YM, et al. Evaluation of European Nutritional Risk Screening method in Chinese hospitalized patients practice. Chin J Clin Nutr 2005;13:137-41.
- Jiang ZM, Chen W, Zhan WH, et al. Parenteral and enteral nutrition application in west, middle and east China: a multicenter investigation for 15098 patients in 13 metropolitans using nutritional risk screening 2002 tool. Clin Nutr Suppl 2007;2:133-4.
- Heyland DK, Montalvo M, MacDonald S, et al. Total parenteral nutrition in the surgical patient: a meta-analysis. Can J Surg 2001;44:102-11. [PubMed]
- Braga M, Gianotti L, Gentilini O, et al. Feeding the gut early after digestive surgery: results of a nine-year experience. Clin Nutr 2002;21:59-65. [PubMed]
- Spies CD, Breuer JP, Gust R, et al. Preoperative fasting. An update. Anaesthesist 2003;52:1039-45. [PubMed]
- Soop M, Nygren J, Thorell A, et al. Preoperative oral carbohydrate treatment attenuates endogenous glucose release 3 days after surgery. Clin Nutr 2004;23:733-41. [PubMed]
- Henriksen MG, Hessov I, Dela F, et al. Effects of preoperative oral carbohydrates and peptides on postoperative endocrine response, mobilization, nutrition and muscle function in abdominal surgery. Acta Anaesthesiol Scand 2003;47:191-9. [PubMed]
- Breuer JP, von Dossow V, von Heymann C, et al. Preoperative oral carbohydrate administration to ASA III-IV patients undergoing elective cardiac surgery. Anesth Analg 2006;103:1099-108. [PubMed]
- Feo CV, Romanini B, Sortini D, et al. Early oral feeding after colorectal resection: a randomized controlled study. ANZ J Surg 2004;74:298-301. [PubMed]
- Nirula R, Yamada K, Waxman K. The effect of abrupt cessation of total parenteral nutrition on serum glucose: a randomized trial. Am Surg 2000;66:866-9. [PubMed]
- Chiu TY, Hu WY, Chuang RB, et al. Terminal cancer patients’ wishes and influencing factors toward the provision of artificial nutrition and hydration in Taiwan. J Pain Symptom Manage 2004;27:206-14. [PubMed]
- Ulander K, Jeppsson B, Grahn G. Postoperative energy intake in patients after colorectal cancer surgery. Scand J Caring Sci 1998;12:131-8. [PubMed]
- Correia MI, Caiaffa WT, da Silva AL, et al. Risk factors for malnutrition in patients undergoing gastroenterological and hernia surgery: an analysis of 374 patients. Nutr Hosp 2001;16:59-64. [PubMed]
- Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med 1991;325:525-32. [PubMed]
- Weimann A, Braga M, Harsanyi L, et al. ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr 2006;25:224-44. [PubMed]
- Takagi K, Yamamori H, Morishima Y, et al. Preoperative immunosuppression: its relationship with high morbidity and mortality in patients receiving thoracic esophagectomy. Nutrition 2001;17:13-7. [PubMed]
- Braga M, Ljungqvist O, Soeters P, et al. ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr 2009;28:378-86. [PubMed]
- Strejc JM. Considerations in the nutritional management of patients with acute renal failure. Hemodial Int 2005;9:135-42. [PubMed]
- Braga M, Gianotti L, Vignali A, et al. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery 2002;132:805-14. [PubMed]
- Jiang H, Jiang ZM, Luo B, et al. An appraisal of immunonutrition for clinical nutritional support with a systematic review of English and Chinese documents. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2002;24:552-8. [PubMed]
- Berger MM, Shenkin A. Vitamins and trace elements: practical aspects of supplementation. Nutrition 2006;22:952-5. [PubMed]
- Lundholm K, Körner U, Gunnebo L, et al. Insulin treatment in cancer cachexia: effects on survival, metabolism, and physical functioning. Clin Cancer Res 2007;13:2699-706. [PubMed]
- Andreyev HJ, Norman AR, Oates J, et al. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 1998;34:503-9. [PubMed]
- Aslani A, Smith RC, Allen BJ, et al. The predictive value of body protein for chemotherapy-induced toxicity. Cancer 2000;88:796-803. [PubMed]
- Bauer JD, Capra S. Nutrition intervention improves outcomes in patients with cancer cachexia receiving chemotherapy--a pilot study. Support Care Cancer 2005;13:270-4. [PubMed]
- Shang E, Weiss C, Post S, et al. The influence of early supplementation of parenteral nutrition on quality of life and body composition in patients with advanced cancer. JPEN J Parenter Enteral Nutr 2006;30:222-30. [PubMed]
- Gramignano G, Lusso MR, Madeddu C, et al. Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition 2006;22:136-45. [PubMed]
- Hasenberg T, Essenbreis M, Herold A, et al. Early supplementation of parenteral nutrition is capable of improving quality of life, chemotherapy-related toxicity and body composition in patients with advanced colorectal carcinoma undergoing palliative treatment: results from a prospective, randomized clinical trial. Colorectal Dis 2010;12:e190-9. [PubMed]
- McCarthy D, Weihofen D. The effect of nutritional supplements on food intake in patients undergoing radiotherapy. Oncol Nurs Forum 1999;26:897-900. [PubMed]
- Arnold C, Richter MP. The effect of oral nutritional supplements on head and neck cancer. Int J Radiat Oncol Biol Phys 1989;16:1595-9. [PubMed]
- Elkort RJ, Baker FL, Vitale JJ, et al. Long-term nutritional support as an adjunct to chemotherapy for breast cancer. JPEN J Parenter Enteral Nutr 1981;5:385-90. [PubMed]
- De Cicco M, Panarello G, Fantin D, et al. Parenteral nutrition in cancer patients receiving chemotherapy: effects on toxicity and nutritional status. JPEN J Parenter Enteral Nutr 1993;17:513-8. [PubMed]
- Bozzetti F, Arends J, Lundholm K, et al. ESPEN Guidelines on Parenteral Nutrition: non-surgical oncology. Clin Nutr 2009;28:445-54. [PubMed]
- Valdivieso M, Frankmann C, Murphy WK, et al. Long-term effects of intravenous hyperalimentation administered during intensive chemotherapy for small cell bronchogenic carcinoma. Cancer 1987;59:362-9. [PubMed]
- Brard L, Weitzen S, Strubel-Lagan SL, et al. The effect of total parenteral nutrition on the survival of terminally ill ovarian cancer patients. Gynecol Oncol 2006;103:176-80. [PubMed]
- Jones L, Watling RM, Wilkins S, et al. Nutritional support in children and young people with cancer undergoing chemotherapy. Cochrane Database Syst Rev 2010;(7):CD003298. [PubMed]
- Märten A, Wente MN, Ose J, et al. An open label randomized multicentre phase IIIb trial comparing parenteral substitution versus best supportive nutritional care in subjects with pancreatic adenocarcinoma receiving 5-FU plus oxaliplatin as 2nd or higher line chemotherapy regarding clinical benefit - PANUSCO. BMC Cancer 2009;9:412. [PubMed]
- Scolapio JS, Fleming CR, Kelly DG, et al. Survival of home parenteral nutrition-treated patients: 20 years of experience at the Mayo Clinic. Mayo Clin Proc 1999;74:217-22. [PubMed]
- de Vries EG, Mulder NH, Houwen B, et al. Enteral nutrition by nasogastric tube in adult patients treated with intensive chemotherapy for acute leukemia. Am J Clin Nutr 1982;35:1490-6. [PubMed]
- Koretz RL, Lipman TO, Klein S, et al. AGA technical review on parenteral nutrition. Gastroenterology 2001;121:970-1001. [PubMed]
- Fini L, Piazzi G, Ceccarelli C, et al. Highly purified eicosapentaenoic acid as free fatty acids strongly suppresses polyps in Apc(Min/+) mice. Clin Cancer Res 2010;16:5703-11. [PubMed]
- Schley PD, Jijon HB, Robinson LE, et al. Mechanisms of omega-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat 2005;92:187-95. [PubMed]
- Dewey A, Baughan C, Dean T, et al. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst Rev 2007;(1):CD004597. [PubMed]
- Jatoi A, Rowland K, Loprinzi CL, et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer-associated wasting: a North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J Clin Oncol 2004;22:2469-76. [PubMed]
- Szymanski KM, Wheeler DC, Mucci LA. Fish consumption and prostate cancer risk: a review and meta-analysis. Am J Clin Nutr 2010;92:1223-33. [PubMed]
- Gogos CA, Ginopoulos P, Salsa B, et al. Dietary omega-3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy: a randomized control trial. Cancer 1998;82:395-402. [PubMed]
- Xue H, Le Roy S, Sawyer MB, et al. Single and combined supplementation of glutamine and n-3 polyunsaturated fatty acids on host tolerance and tumour response to 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxy-camptothecin (CPT-11)/5-fluorouracil chemotherapy in rats bearing Ward colon tumour. Br J Nutr 2009;102:434-42. [PubMed]
- Ng K, Meyerhardt JA, Chan JA, et al. Multivitamin use is not associated with cancer recurrence or survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol 2010;28:4354-63. [PubMed]
- Polisena CG, Wade VR. Cancer patients need referrals to dietitians. J Am Diet Assoc 1993;93:975-6. [PubMed]
- Arends J, Bodoky G, Bozzetti F, et al. ESPEN Guidelines on Enteral Nutrition: Non-surgical oncology. Clin Nutr 2006;25:245-59. [PubMed]
- Bozzetti F, Arends J, Lundholm K, et al. ESPEN Guidelines on Parenteral Nutrition: non-surgical oncology. Clin Nutr 2009;28:445-54. [PubMed]
- Daly JM, Weintraub FN, Shou J, et al. Enteral nutrition during multimodality therapy in upper gastrointestinal cancer patients. Ann Surg 1995;221:327-38. [PubMed]
- Fietkau R, Iro H, Sailer D, et al. Percutaneous endoscopically guided gastrostomy in patients with head and neck cancer. Recent Results Cancer Res 1991;121:269-82. [PubMed]
- Thiel HJ, Fietkau R, Sauer R. Malnutrition and the role of nutritional support for radiation therapy patients. Recent Results Cancer Res 1988;108:205-26. [PubMed]
- Langius JA, Doornaert P, Spreeuwenberg MD, et al. Radiotherapy on the neck nodes predicts severe weight loss in patients with early stage laryngeal cancer. Radiother Oncol 2010;97:80-5. [PubMed]
- Isenring EA, Capra S, Bauer JD. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br J Cancer 2004;91:447-52. [PubMed]
- van den Berg MG, Rasmussen-Conrad EL, Wei KH, et al. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr 2010;104:872-7. [PubMed]
- Ravasco P, Monteiro-Grillo I, Vidal PM, et al. Dietary counseling improves patient outcomes: a prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J Clin Oncol 2005;23:1431-8. [PubMed]
- Lee JH, Machtay M, Unger LD, et al. Prophylactic gastrostomy tubes in patients undergoing intensive irradiation for cancer of the head and neck. Arch Otolaryngol Head Neck Surg 1998;124:871-5. [PubMed]
- Marcy PY, Magné N, Bensadoun RJ, et al. Systematic percutaneous fluoroscopic gastrostomy for concomitant radiochemotherapy of advanced head and neck cancer: optimization of therapy. Support Care Cancer 2000;8:410-3. [PubMed]
- Tyldesley S, Sheehan F, Munk P, et al. The use of radiologically placed gastrostomy tubes in head and neck cancer patients receiving radiotherapy. Int J Radiat Oncol Biol Phys 1996;36:1205-9. [PubMed]
- Beer KT, Krause KB, Zuercher T, et al. Early percutaneous endoscopic gastrostomy insertion maintains nutritional state in patients with aerodigestive tract cancer. Nutr Cancer 2005;52:29-34. [PubMed]
- Mangar S, Slevin N, Mais K, et al. Evaluating predictive factors for determining enteral nutrition in patients receiving radical radiotherapy for head and neck cancer: a retrospective review. Radiother Oncol 2006;78:152-8. [PubMed]
- Rabinovitch R, Grant B, Berkey BA, et al. Impact of nutrition support on treatment outcome in patients with locally advanced head and neck squamous cell cancer treated with definitive radiotherapy: a secondary analysis of RTOG trial 90-03. Head Neck 2006;28:287-96. [PubMed]
- Scolapio JS, Tarrosa VB, Stoner GL, et al. Audit of nutrition support for hematopoietic stem cell transplantation at a single institution. Mayo Clin Proc 2002;77:654-9. [PubMed]
- Paccagnella A, Morello M, Da Mosto MC, et al. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support Care Cancer 2010;18:837-45. [PubMed]
- Koretz RL, Lipman TO, Klein S, et al. AGA technical review on parenteral nutrition. Gastroenterology 2001;121:970-1001. [PubMed]
- Freedman B. Equipoise and the ethics of clinical research. N Engl J Med 1987;317:141-5. [PubMed]
- Loiudice TA, Lang JA. Treatment of radiation enteritis: a comparison study. Am J Gastroenterol 1983;78:481-7. [PubMed]
- Scolapio JS, Fleming CR, Kelly DG, et al. Survival of home parenteral nutrition-treated patients: 20 years of experience at the Mayo Clinic. Mayo Clin Proc 1999;74:217-22. [PubMed]
- Bozzetti F. Home parenteral nutrition. CAB International Publ 2006:95-102.
- Shang E, Weiss C, Post S, et al. The influence of early supplementation of parenteral nutrition on quality of life and body composition in patients with advanced cancer. JPEN J Parenter Enteral Nutr 2006;30:222-30. [PubMed]
- Collins MM, Wight RG, Partridge G. Nutritional consequences of radiotherapy in early laryngeal carcinoma. Ann R Coll Surg Engl 1999;81:376-81. [PubMed]
- Strang P. The effect of megestrol acetate on anorexia, weight loss and cachexia in cancer and AIDS patients. Anticancer Res 1997;17:657-62. [PubMed]
- Fearon KC, Voss AC, Hustead DS, et al. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 2006;83:1345-50. [PubMed]
- Lundholm K, Daneryd P, Bosaeus I, et al. Palliative nutritional intervention in addition to cyclooxygenase and erythropoietin treatment for patients with malignant disease: Effects on survival, metabolism, and function. Cancer 2004;100:1967-77. [PubMed]
- Li JS. Individualized nutritional therapy for critically ill patients. Parenteral & Enteral Nutrition 2009;16:193-4.
- Bozzetti F, Arends J, Lundholm K, et al. ESPEN Guidelines on Parenteral Nutrition: non-surgical oncology. Clin Nutr 2009;28:445-54. [PubMed]
- Huhmann MB, August DA. Review of American Society for Parenteral and Enteral Nutrition (ASPEN) Clinical Guidelines for Nutrition Support in Cancer Patients: nutrition screening and assessment. Nutr Clin Pract 2008;23:182-8. [PubMed]
- Ruiz-García V, Juan O, Pérez Hoyos S, et al. Megestrol acetate: a systematic review usefulness about the weight gain in neoplastic patients with cachexia. Med Clin (Barc) 2002;119:166-70. [PubMed]
- Babcock T, Helton WS, Espat NJ. Eicosapentaenoic acid (EPA): an antiinflammatory omega-3 fat with potential clinical applications. Nutrition 2000;16:1116-8. [PubMed]
- Gao JQ, Wu GH, Yuan L, et al. Effects of ω-3 polyunsaturated fatty acid on the nutritional status of tumor-bearing rats. Journal of Surgery Concepts & Practice 2008;13:419-22.
- Ferreri NR, McGiff JC, Carroll MA, et al. Renal COX-2, cytokines and 20-HETE: tubular and vascular mechanisms. Curr Pharm Des 2004;10:613-26. [PubMed]
- Gordon JN, Trebble TM, Ellis RD, et al. Thalidomide in the treatment of cancer cachexia: a randomised placebo controlled trial. Gut 2005;54:540-5. [PubMed]
- MacDonald N, Easson AM, Mazurak VC, et al. Understanding and managing cancer cachexia. J Am Coll Surg 2003;197:143-61. [PubMed]


