Animal models of meningiomas
Introduction
Meningiomas are the most frequent intracranial neoplasms. Meningiomas are tumors of the elderly, with a clearly increased incidence after the age of 65 years (1). Meningiomas preferentially affect women with a female: male ration of 3.5:1 (2). Other risk factors are ionizing radiation, diabetes mellitus, hypertension, and possibly smoking (3-6). Meningiomas in children and young adults are rare, however, in patients suffering from germline mutations in the neurofibromatosis type 2 (NF2) gene, single or even multiple meningiomas may be present (7).
Approximately 90% of meningiomas arise in the cranial meninges, while 10% occur in the spinal meninges. Within the cranial cavity, sites of meningioma growth can be separated into tumors of the convexity and tumors growing in the anterior, middle, or posterior skull base. Especially skull base meningiomas may cause considerable morbidity and may be challenging for the neurosurgeon, and the clinical course is at least partly dependent on the localization of the tumor (8,9).
While the tumor resection by the neurosurgeon is regarded as standard therapy for meningiomas, a fraction of tumors may be resected only incompletely, with subsequent tumor regrowth and/or recurrence. Moreover, meningiomas in difficult locations may be not eligible for resection. In these cases or in patients with recurrent tumors, additional treatment options may be required. Another limitation for surgery might be caused by the overall health condition of the patient or other co-existing diseases, a scenario which is not infrequent within the typical meningioma patient age group.
As an additional treatment option for aggressive, recurrent or inaccessible meningiomas, radiotherapy is recommended [reviewed in (10)]. In aggressive meningioma subtypes, radiotherapy can achieve good results (11).
Beside radiotherapy, additional medical treatment for meningiomas has shown only limited efficacy so far (12). This is based partly on the limited knowledge regarding molecular alterations with relevance to treatment in meningiomas, but also on the lack of animal models with gene-specific alterations, covering the spectrum of known or supposed driver mutations, to study treatment efficacy.
Meningiomas are characterized by a high diversity of histopathological features. The dominating histological subtypes among the WHO (World Health Organization) grade I meningiomas are the meningothelial and fibroblastic subtype, or a mixture from both designated as transitional meningiomas. While most of the meningioma subtypes belong to the WHO grade I, about 20 percent are diagnosed as atypical or anaplastic meningiomas (13). Grade II and grade III meningiomas have substantial impact on morbidity and mortality (14). These tumors display aggressive biological features with high proliferation activity and infiltrative growth. In the recently updated WHO classification of brain tumors, brain invasion by meningiomas qualify them for the grade II designation (15).
Genetic alterations in meningiomas as basis for model development
The molecular biology of meningiomas is complex and determined by the age group affected, the localization of the tumor, and the histological subtype. The key molecular alterations present in sporadic meningiomas are allelic losses and/or mutations in the NF2 gene at chromosome 22q (16,17). NF2 alterations are preferentially found in meningiomas of the convexity and of fibroblastic/transitional subtype. In patients suffering from NF2, multiple meningiomas may arise in children or young adults. Vice versa, occurrence of a meningioma during childhood is a strong indicator for the presence of NF2 (18). Recently, a number of other genetic alterations have been identified mostly on NF2-wild type intracranial meningiomas. They affect the genes Smo, AKT1, TRAF7, KLF4, POLR2A, PI3K, SMARCB1 and BAP1. The frequency of these alterations is much lower than the one seen for NF2, but they show a surprising preference for certain localizations or histological subtypes (19-26). Additionally, for meningiomas with clear-cell WHO-grade II subtype located either intracranially, or in the majority of cases in the spinal cord, mutations in the SMARCE1-Gen have been identified as tumor-initiating event (27). However, the modeling especially of the newly-identified genetic alterations to evaluate the tumor-initiating or at least tumor-accelerating properties has not been developed thus far.
Meningioma modeling: questions and challenges
Based on the various clinical, pathological, molecular, and therapeutical characteristics and challenges associated with meningiomas, appropriate modeling requires addressing of a number of key issues which are listed in Table 1.
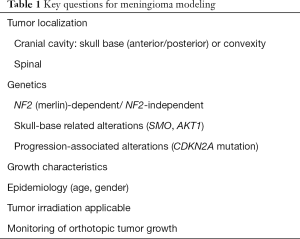
Full table
The first point is related to the NF2 gene. Given the high frequency of NF2 alterations in sporadic meningiomas, understanding growth characteristics and treatment efficacy should include this essential gene. NF2 (with the gene product merlin) is a tumor suppressor and a member of the 4.1/FERM gene family (4.1, ezrin, radixin, moesin). FERM domain proteins are linker between plasma membrane receptors and the actin cytoskeleton (28). Merlin regulates receptor-mediated signaling processes essential for cell proliferation, cell adhesion, and survival. Relevant pathways affected by merlin include PI3K/Akt, mTOR (mammalian target of rapamycin), small GTPases, and the hippo pathway [reviewed in (29)]. Merlin action might therefore affect all drug-based treatment options targeting one of the above-mentioned pathways.
Another aspect is related to the tumor location. Beside the fact that the frequency of certain molecular alterations is related to the localization of the tumor (convexity, skull base, spinal cord), the neurosurgical treatment especially for skull base meningiomas can be challenging (9). Therefore, skull base-located tumors are prone to incomplete resection and subsequent early tumor recurrence. It is well-known that certain histological subtypes preferentially occur at certain sites. For instance, psammomatous or clear cell meningiomas are frequently found within the spinal cord, while meningothelial meningiomas favor the skull base and fibroblastic meningiomas the convexity (10). Embryonically, the meninges at the skull base are derived from the mesoderm, while telencephalic meninges are neural crest-derived, explaining the different histological subtype development at least partly (30). Therefore, tumor location is relevant both from a clinical and biological point of view.
The site of tumor growth in a given meningioma model is moreover relevant for the potential application of radiation therapy, which can potential enhance drug sensitivity (31). Meningiomas growing at the convexity are easier accessible for irradiation then meningiomas growing at the skull-base in an animal model. This tackles the question of monitoring tumor growth in model systems in addition, because meningiomas growing at the convexity might be monitored by luciferase-base techniques, while skull-base related models require magnetic resonance imaging (MRI) examination for follow-up.
Finally, most meningiomas are naturally slow-growing tumors which develop in elderly patients (32,33). There seems to be a phase of minimal growth, but over the age of 60 years an accelerated tumor growth may occur (32). Although the tumor initiation within a very restricted time window after birth have been demonstrated in mice (34) (see below), it is unclear why meningiomas need a surprisingly long time until relevant tumor growth is going to be started. Taken together, meningioma modeling is complicated by a plethora of different clinical and biological features.
Genetically-based meningioma models
Driven by the observation that NF2 inactivation is frequently found in sporadic meningiomas, and that in patients with NF2 meningiomas arise early in life and sometimes in multiplicity, the first convincing meningioma model using genetically-engineered mice (GEMM) has targeted the NF2 gene.
Michel Kalamarides and his group demonstrated that Nf2 inactivation in leptomeningeal cells of conditional Nf2 knockout mice (Nf2flox/flox) by Cre-recombinase injection induces meningiomas (34). The injection of Cre-recombinase was performed in two ways: intraorbitally, or subdural injection into the frontal convexity area of newborn pubs. Most of these mouse tumors they could induce recapitulated the meningothelial, fibroblastic, or transitional subtype of human meningiomas, and there characterized by reduced merlin expression. However, the tumor induction was restricted to a narrow window of Cre-recombinase injection around postnatal day 2–3. Moreover, the efficiency of tumor induction was limited (29% for transorbital induction and 19% for convexity injection). Regarding the tumor burden, mice died beginning at the age of approximately 8 months. These features indicate that despite the proof for a fundamental role of NF2 in meningioma induction, other factors might operate to end up with the frequency of ~50% sporadic meningiomas with NF2 alterations in humans. The authors could indeed shorten the time to tumor induction and death of mice by introducing with heterozygous p53 loss (Nf2flox/floxp53+/−). However, TP53 alterations are rare in human meningiomas (35,36), indicating that additional genes might be affected. This was further elucidated in a subsequent study by the same group, addressing the question whether inactivation of the Cdkn2ab gene in mice might accelerate the meningioma formation. The CDKN2A gene with its gene product p16 is frequently altered during meningioma progression; alterations on chromosome 9p21 during meningioma progression have been found to represent losses of the tumor suppressor genes CDKN2A (p16INK4a), p14ARF, and CDKN2B (p15INK4b) (37,38). In anaplastic grade III meningiomas, deletions of CDKN2A/CDKN2B are associated with reduced survival (39). The group from Michel Kalamarides indeed could show that additional deletion of Cdkn2a, together with Nf2 inactivation, results in increased meningioma frequency and development of grade II/grade III meningiomas in mice, proving that loss of CDKN2A and CDKN2B is a feature for aggressive meningioma development (40,41).
As mentioned above, the meningothelial and fibroblastic subtype are the dominating histological subtypes among the WHO grade I meningiomas. Thus, it would be interesting to model the development of these major subtypes in mice. The knowledge regarding the development of WHO grade I-subtypes could be improved recently by generating a mouse model based on inactivation of meningeal NF2 by using the prostaglandin D2 synthase (PGDS) gene promoter. PGDS is a specific marker of arachnoidal cells (42). It was nicely shown that Nf2 inactivation in PDGS-positive meningeal progenitor cells was capable to give rise to both meningothelial and fibroblastic meningiomas in 38% of mice (43).
One of the key mitogens in meningiomas is platelet-derived growth factor (PDGF) (44,45). Using the PGDS system again, the group from Michel Kalamarides could demonstrate that PDGF overexpression in arachnoidal cells using the RCAS-TVA system (46) could induce meningiomas independent from Nf2 inactivation (47). Furthermore, malignant transformation of meningiomas could be modeled by combined Nf2 inactivation and PDGF overexpression.
Taken together, using mice with floxed Nf2 (including the use of PGDS as a meningeal promoter) and meningeal inactivation of Nf2 it could be established that NF2 is critical for the induction of a relevant fraction of meningiomas, and that meningioma aggressiveness can be recapitulated by additional CDKN2ab inactivation. Based on the site of NF2 inactivation, both predominating histopathological meningioma subtypes can be generated. Unfortunately, the induction of non-NF2-based meningiomas is not well understood. This is especially relevant because several new genes have been identified in NF2 wild-type meningiomas (SMO, KLF4, TRAF7, AKT1), while their potential as true meningioma tumor drivers is unclear so far. Moreover, some drugs targeting these mutations have shown promising results, as recently demonstrated for the AKT inhibitor AZD5363 successfully administered in a patient with metastatic AKT1-mutated meningioma (48).
Xenograft models using patient-derived tumor tissue and meningioma cell lines
As shown before, the GEM model available so far have some disadvantages, including the low rate of tumor induction and the long time necessary until substantial tumor growth is recognized. The latter further complicates the evaluation of potential medical treatment strategies.
Implantation of human tumor cells into immune-compromized (“nude”) mice is a well-established method to evaluate tumorigenicity and potential treatment efficacy (49). For meningiomas, however, the human tumor cells need to be delivered at sites prone to develop meningiomas in order to respect the environmental factors which modulate tumor growth. These environmental factors include cerebral spinal fluid, arachnoidal, brain, or bone tissue, as well as space differences evident between convexity, skull base, and spinal cord, respectively. Moreover, implantation of cells should not be limited by substantial animal mortality or morbidity do to the operation procedure itself.
There are several papers reporting the implantation of primary meningioma cells or cell lines into the flank of nude mice for monitoring meningioma tumor cell growth and potential treatment effects [for instance (50-54)]. Generally, this process is enhanced by matrigel-based tumor cell formation (55). However, it is debatable whether a subcutaneous flank model can serve as a real meningioma model or not. Thus, details of flank model-derived data will not be discussed in detail in this review.
Characteristics of meningioma cell lines used for xenograft experiments
The use of primary meningioma cultures is restricted to early passages due to cellular senescence, but expression of the telomerase catalytic subunit (hTERT), together with expression of the human papillomavirus E6 and E7 oncogenes in cells derived from WHO grade I tumors, may overcome this limitation (56). On the other hand, some established cell lines derived from highly aggressive meningioma variants are available, and the majority of xenograft studies indeed are using the latter. Table 2 gives an overview about the essential characteristics of widely used meningioma cell lines.

Full table
The best characterized line derived from a benign WHO grade I meningioma is the line BenMen1 (57). The cells are derived from a WHO grade I meningothelial meningioma and show a loss of chromosome 22. The majority of the other reported cell lines used for orthotopic xenograft studies are derived from anaplastic malignant meningiomas, while the line IOMM-Lee unfortunately does not contain the typical loss of chromosome 22q (58) (Table 2). The other malignant lines have unlimited growth potential and harbor alterations of the NF2 gene at chromosome 22, making them eligible for meningioma studies with inclusion of the characteristic NF2 alterations seen in sporadic meningiomas.
A major limitation of these cell lines is based on the fact that no NF2 wild-type control cell is available. Therefore, it would be desirable to generate (ideally) patient-derived cell lines from NF2 wild-type tumors with unlimited growth and subsequent inactivation of NF2. Indeed, these cell pairs have been generated in the past. Striedinger et al. (61) reported a cell pair called MENII-1, which is derived from a WHO grade II meningioma and have been immortalized by E6/E7 human papilloma genes. These cells were used to knockdown NF2/merlin using NF2-siRNA constructs, yielding a genetically comparable pair of MENII cells with and without merlin expression. However, the usefulness of these paired cells for orthotopic xenografts to model the effect of merlin loss for meningioma growth is unclear. The same group reported the alternative strategy in the same paper (61): malignant KT21 cells with chromosome 22q loss (Table 2) were used to overexpress wild-type NF2, with successful re-expression of the NF2 gene product merlin. However, studies using pairwise NF2 wild type/NF2 mutant cells with stable merlin loss or merlin overexpression have been not been reported in the context of mouse xenografts.
Cerebral convexity and skull base models
Implantation of primary, non-manipulated cells derived from benign meningiomas was demonstrated to result in a reliable growth of tumors (93% of mice with implanted tumors) after 3 months (62). In this approach, tumor cells were implanted into the convexity of the prefrontal cortex. The tumors exhibited showed typical meningioma features, and in a subsequent study this model was used to test whether celecoxib treatment might affect tumor growth, with only limited success (63). Implantation of meningioma cells directly below the skull to model meningiomas of the convexity have been reported from several groups. Cargioli et al. (54) generated two different meningioma cell lines (Me3TSC and Me10T) by hTERT immortalization and observed after a period of 16 weeks that all mice with injected meningioma cells harbored meningioma-like tumors. Michelhaugh et al. (64) generated a cell line from a WHO grade I meningioma which was spontaneously immortal and observed orthotopic tumor growth, while the observation time of these animals was not described in detail. Our group has performed several studies with injection of IOMM-Lee cells for modeling convexity meningiomas, and animals were subsequently treated with different systemic drugs either by intraperitoneal injection, or by oral application. Normally, untreated mice are alive for 12–16 days in this model, while significant extension of survival can be achieved by systemic drug treatment (31,65,66). Moreover, the explanted tumors can be used for biochemical analyses, mostly immunohistochemistry and western blotting, to prove the orthotopic downregulation of the desired drug target. However, a major drawback is the fact that the tumor growth of IOMM-Lee cells is fast, prohibiting longer observation periods with ongoing treatment, or after cessation of drug application. Recently, xenografts (tumorspheres) derived from malignant meningioma were implanted into the convexity and successfully treated with oncolytic herpes simplex virus by another group, with a surprisingly low number of implanted cells necessary for tumor induction (67). It should be mentioned that convexity-based xenograft models open the opportunity to irradiate the tumor in order to evaluate possible radiation-associated treatment augmentation (31).
To model growth of meningiomas at the skull-base, a number of studies have been published. Earliest reports showed successful growth at both convexity and skull base of immortalized cell lines (CH-157-MN and IOMM-Lee). Tumor growth was successful in the majority of implantation procedures (68). Other groups have implanted meningioma cells (IOMM-Lee) into the region of the temporal fossa to model the growth of skull base meningiomas (69,70). In one study, mice with implanted IOMM-Lee cells at the skull base were treated with Temozolomide, and significant survival benefit was seen (71). In general, a major disadvantage of xenograft models is the dependency on an immunocompromised host, deleting potential important immune-cell based antitumor processes. Moreover, the growth characteristics may not recapitulate the situation in humans completely, and it is not completely clear how closely related the immortal meningioma cell lines are in fact to the human tumors. Table 3 summarizes the key features of orthotopic meningioma xenograft models.

Full table
Monitoring of mouse meningioma tumor growth
It is highly desirable to have monitoring options for meningioma mouse models available. In case of GEMM, the rate of tumor induction per se needs to be evaluated, and, in case of successful tumor induction, growth rate and potential treatment effects can be followed. For xenograft models, the induction rate is usually high, but a true orthotopic growth and potential treatment effects need to be evaluated. Two principle ways are available: imaging using small-animal MRI, and bioluminescence-based (BL) methods.
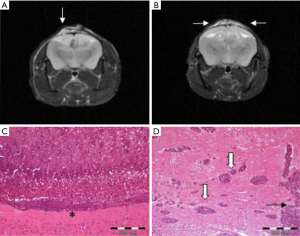
MR imaging to detect the rate of tumor induction in GEMM for meningiomas have been reported by Kalamarides et al. (34). Additionally, the same group has successfully applied BL to detect the growth of genetically-induced meningioma (40). The most widely used cells for xenograft imaging are IOMM-Lee and KT21 (71,72). MRI monitoring as a valuable tool for monitoring of growth and treatment response has been reported by several groups (64,66,77,78) (Figure 1). On the other hand, a less invasive (and less expensive) method is the use of BL for cells which have been labeled before with GFP (green fluorescent protein) or other fluorescence dyes. Iwami and colleagues (79) used green fluorescence (GFP)-labeled IOMM-lee cells and could show nice tumor growth within 14 days. Both MRI and BL surveillance is an essential requirement for the study of meningioma growth in mice.
Conclusions
Mouse models, either based on the inactivation of specific meningioma-associated genes, or based on orthotopic implantation of human xenograft meningioma cells in mouce, provide valuable insight into growth kinetics and treatment effects. Incorporation of newly identified genes altered in meningiomas in both models is challenging, but will enhance the knowledge about the impact of these genes and their potential treatment. For an overall assessment of a mouse meningioma model, all relevant clinical features of meningiomas should be considered.
Acknowledgements
I am thankful to Markus Tuchen for providing the mouse MRI picture.
Funding: Meningioma research of the author is supported by grants from the DFG (grant #MA2530/6-1 and #MA2530/8-1), the Wilhelm Sander-Stiftung (grant #2014.092.1), and the Deutsche Krebshilfe (grant #111853).
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol 2015;17:iv1-iv62. [Crossref] [PubMed]
- Klaeboe L, Lonn S, Scheie D, et al. Incidence of intracranial meningiomas in Denmark, Finland, Norway and Sweden, 1968-1997. Int J Cancer 2005;117:996-1001. [Crossref] [PubMed]
- Flint-Richter P, Mandelzweig L, Oberman B, et al. Possible interaction between ionizing radiation, smoking, and gender in the causation of meningioma. Neuro Oncol 2011;13:345-52. [Crossref] [PubMed]
- Sadetzki S, Flint-Richter P, Ben-Tal T. Radiation induced meningioma: a descriptive study of 253 cases. J Neurosurg 2002;97:1078-82. [Crossref] [PubMed]
- Schneider B, Pulhorn H, Rohrig B, et al. Predisposing conditions and risk factors for development of symptomatic meningioma in adults. Cancer detection and prevention 2005;29:440-7. [Crossref] [PubMed]
- Niedermaier T, Behrens G, Schmid D, et al. Body mass index, physical activity, and risk of adult meningioma and glioma: A meta-analysis. Neurology 2015;85:1342-50. [Crossref] [PubMed]
- Perry A, Giannini C, Raghavan R, et al. Aggressive phenotypic and genotypic features in pediatric and NF2-associated meningiomas: a clinicopathologic study of 53 cases. J Neuropathol Exp Neurol 2001;60:994-1003. [Crossref] [PubMed]
- Nakamura M, Roser F, Jacobs C, et al. Medial sphenoid wing meningiomas: clinical outcome and recurrence rate. Neurosurgery 2006;58:626-39, discussion 626-39. [Crossref] [PubMed]
- Nakamura M, Struck M, Roser F, et al. Olfactory groove meningiomas: clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery 2007;60:844-52; discussion 844-52. [Crossref] [PubMed]
- Mawrin C, Chung C, Preusser M. Biology and clinical management challenges in meningioma. Am Soc Clin Oncol Educ Book 2015.e106-15. [Crossref] [PubMed]
- Milosevic MF, Frost PJ, Laperriere NJ, et al. Radiotherapy for atypical or malignant intracranial meningioma. Int J Radiat Oncol Biol Phys 1996;34:817-22. [Crossref] [PubMed]
- Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 2016;17:e383-91. [Crossref] [PubMed]
- Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol 2010;99:379-91. [Crossref] [PubMed]
- Perry A, Scheithauer BW, Stafford SL, et al. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer 1999;85:2046-56. [PubMed]
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Ruttledge MH, Sarrazin J, Rangaratnam S, et al. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat Genet 1994;6:180-4. [Crossref] [PubMed]
- Ruttledge MH, Xie YG, Han FY, et al. Deletions on chromosome 22 in sporadic meningioma. Genes Chromosomes Cancer 1994;10:122-30. [Crossref] [PubMed]
- Evans DG, Watson C, King A, et al. Multiple meningiomas: differential involvement of the NF2 gene in children and adults. J Med Genet 2005;42:45-8. [Crossref] [PubMed]
- Clark VE, Erson-Omay EZ, Serin A, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013;339:1077-80. [Crossref] [PubMed]
- Clark VE, Harmanci AS, Bai H, et al. Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nat Genet 2016;48:1253-9. [Crossref] [PubMed]
- Brastianos PK, Horowitz PM, Santagata S, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet 2013;45:285-9. [Crossref] [PubMed]
- Reuss DE, Piro RM, Jones DT, et al. Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations. Acta Neuropathol 2013;125:351-8. [Crossref] [PubMed]
- Sahm F, Bissel J, Koelsche C, et al. AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry. Acta Neuropathologica 2013;126:757-62. [Crossref] [PubMed]
- Abedalthagafi M, Bi WL, Aizer AA, et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro Oncol 2016;18:649-55. [Crossref] [PubMed]
- Smith MJ. Germline and somatic mutations in meningiomas. Cancer genetics 2015;208:107-14. [Crossref] [PubMed]
- Shankar GM, Abedalthagafi M, Vaubel RA, et al. Germline and somatic BAP1 mutations in high-grade rhabdoid meningiomas. Neuro Oncol 2017;19:535-45. [PubMed]
- Smith MJ, Wallace AJ, Bennett C, et al. Germline SMARCE1 mutations predispose to both spinal and cranial clear cell meningiomas. J Pathol 2014;234:436-40. [Crossref] [PubMed]
- Trofatter JA, MacCollin MM, Rutter JL, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell 1993;72:791-800. [Crossref] [PubMed]
- Stamenkovic I, Yu Q. Merlin, a “Magic” Linker Between the Extracellular Cues and Intracellular Signaling Pathways that Regulate Cell Motility, Proliferation, and Survival. Current protein & peptide science 2010;11:471-84. [Crossref] [PubMed]
- Catala M. Embryonic and fetal development of structures associated with the cerebro-spinal fluid in man and other species. Part I: The ventricular system, meninges and choroid plexuses. Arch Anat Cytol Pathol 1998;46:153-69. [PubMed]
- Wilisch-Neumann A, Kliese N, Pachow D, et al. The Integrin Inhibitor Cilengitide Affects Meningioma Cell Motility and Invasion. Clin Cancer Res 2013;19:5402-12. [Crossref] [PubMed]
- Nakamura M, Roser F, Michel J, et al. The natural history of incidental meningiomas. Neurosurgery 2003;53:62-70; discussion 70-1. [Crossref] [PubMed]
- Oya S, Kim SH, Sade B, et al. The natural history of intracranial meningiomas. J Neurosurg 2011;114:1250-6. [Crossref] [PubMed]
- Kalamarides M, Niwa-Kawakita M, Leblois H, et al. Nf2 gene inactivation in arachnoidal cells is rate-limiting for meningioma development in the mouse. Genes & development 2002;16:1060-5. [Crossref] [PubMed]
- Weber RG, Bostrom J, Wolter M, et al. Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: toward a genetic model of meningioma progression. Proc Natl Acad Sci U S A 1997;94:14719-24. [Crossref] [PubMed]
- Joachim T, Ram Z, Rappaport ZH, et al. Comparative analysis of the NF2, TP53, PTEN, KRAS, NRAS and HRAS genes in sporadic and radiation-induced human meningiomas. Int J Cancer 2001;94:218-21. [Crossref] [PubMed]
- Boström J, Meyer-Puttlitz B, Wolter M, et al. Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am J Pathol 2001;159:661-9. [Crossref] [PubMed]
- Goutagny S, Yang HW, Zucman-Rossi J, et al. Genomic profiling reveals alternative genetic pathways of meningioma malignant progression dependent on the underlying NF2 status. Clin Cancer Res 2010;16:4155-64. [Crossref] [PubMed]
- Perry A, Banerjee R, Lohse CM, et al. A role for chromosome 9p21 deletions in the malignant progression of meningiomas and the prognosis of anaplastic meningiomas. Brain Pathol 2002;12:183-90. [PubMed]
- Peyre M, Stemmer-Rachamimov A, Clermont-Taranchon E, et al. Meningioma progression in mice triggered by Nf2 and Cdkn2ab inactivation. Oncogene 2013;32:4264-72. [Crossref] [PubMed]
- Kalamarides M, Stemmer-Rachamimov AO, Takahashi M, et al. Natural History of Meningioma Development in Mice Reveals: A Synergy of Nf2 and p16Ink4a Mutations. Brain Pathology 2008;18:62-70. [Crossref] [PubMed]
- Kawashima M, Suzuki SO, Yamashima T, et al. Prostaglandin D synthase (beta-trace) in meningeal hemangiopericytoma. Mod Pathol 2001;14:197-201. [Crossref] [PubMed]
- Kalamarides M, Stemmer-Rachamimov AO, Niwa-Kawakita M, et al. Identification of a progenitor cell of origin capable of generating diverse meningioma histological subtypes. Oncogene 2011;30:2333-44. [Crossref] [PubMed]
- Figarella-Branger D, Vagner-Capodano AM, Bouillot P, et al. Platelet-derived growth factor (PDGF) and receptor (PDGFR) expression in human meningiomas: correlations with clinicopathological features and cytogenetic analysis. Neuropathol Appl Neurobiol 1994;20:439-47. [Crossref] [PubMed]
- Mawrin C, Sasse T, Kirches E, et al. Different activation of mitogen-activated protein kinase and Akt signaling is associated with aggressive phenotype of human meningiomas. Clin Cancer Res 2005;11:4074-82. [Crossref] [PubMed]
- Hambardzumyan D, Parada LF, Holland EC, et al. Genetic modeling of gliomas in mice: new tools to tackle old problems. Glia 2011;59:1155-68. [Crossref] [PubMed]
- Peyre M, Salaud C, Clermont-Taranchon E, et al. PDGF activation in PGDS-positive arachnoid cells induces meningioma formation in mice promoting tumor progression in combination with Nf2 and Cdkn2ab loss. Oncotarget 2015;6:32713-22. [PubMed]
- Weller M, Roth P, Sahm F, et al. Durable control of metastatic AKT1-mutant WHO-grade I meningothelial meningioma by the AKT inhibitor, AZD5363. J Natl Cancer Inst 2017;109:1-4. [Crossref] [PubMed]
- Shultz LD, Goodwin N, Ishikawa F, et al. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb Protoc 2014;2014:694-708. [Crossref] [PubMed]
- Schrell UM, Rittig MG, Anders M, et al. Hydroxyurea for treatment of unresectable and recurrent meningiomas. I. Inhibition of primary human meningioma cells in culture and in meningioma transplants by induction of the apoptotic pathway. J Neurosurg 1997;86:845-52. [Crossref] [PubMed]
- Ragel BT, Gillespie DL, Kushnir V, et al. Calcium channel antagonists augment hydroxyurea- and ru486-induced inhibition of meningioma growth in vivo and in vitro. Neurosurgery 2006;59:1109-20; discussion 20-1. [Crossref] [PubMed]
- Ragel BT, Jensen RL, Gillespie DL, et al. Celecoxib inhibits meningioma tumor growth in a mouse xenograft model. Cancer 2007;109:588-97. [Crossref] [PubMed]
- Gupta V, Su YS, Samuelson CG, et al. Irinotecan: a potential new chemotherapeutic agent for atypical or malignant meningiomas. J Neurosurg 2007;106:455-62. [Crossref] [PubMed]
- Cargioli TG, Ugur HC, Ramakrishna N, et al. Establishment of an in vivo meningioma model with human telomerase reverse transcriptase. Neurosurgery 2007;60:750-9; discussion 9-60. [Crossref] [PubMed]
- Jensen RL, Leppla D, Rokosz N, et al. Matrigel augments xenograft transplantation of meningioma cells into athymic mice. Neurosurgery 1998;42:130-5; discussion 5-6. [Crossref] [PubMed]
- Baia GS, Slocum AL, Hyer JD, et al. A genetic strategy to overcome the senescence of primary meningioma cell cultures. J Neurooncol 2006;78:113-21. [Crossref] [PubMed]
- Püttmann S, Senner V, Braune S, et al. Establishment of a benign meningioma cell line by hTERT-mediated immortalization. Lab Invest 2005;85:1163-71. [Crossref] [PubMed]
- Lee WH. Characterization of a newly established malignant meningioma cell line of the human brain: IOMM-Lee. Neurosurgery 1990;27:389-95; discussion 96. [Crossref] [PubMed]
- Yazaki T, Takamiya Y, Costello PC, et al. Inhibition of angiogenesis and growth of human non-malignant and malignant meningiomas by TNP-470. J Neurooncol 1995;23:23-9. [Crossref] [PubMed]
- Tanaka K, Sato C, Maeda Y, et al. Establishment of a human malignant meningioma cell line with amplified c-myc oncogene. Cancer 1989;64:2243-9. [Crossref] [PubMed]
- Striedinger K, Vandenberg SR, Baia GS, et al. The neurofibromatosis 2 tumor suppressor gene product, merlin, regulates human meningioma cell growth by signaling through YAP. Neoplasia 2008;10:1204-12. [Crossref] [PubMed]
- Friedrich S, Schwabe K, Klein R, et al. Comparative morphological and immunohistochemical study of human meningioma after intracranial transplantation into nude mice. J Neurosci Methods 2012;205:1-9. [Crossref] [PubMed]
- Friedrich S, Schwabe K, Grote M, et al. Effect of systemic celecoxib on human meningioma after intracranial transplantation into nude mice. Acta Neurochir (Wien) 2013;155:173-82. [Crossref] [PubMed]
- Michelhaugh SK, Guastella AR, Varadarajan K, et al. Development of patient-derived xenograft models from a spontaneously immortal low-grade meningioma cell line, KCI-MENG1. J Transl Med 2015;13:227. [Crossref] [PubMed]
- Tuchen M, Wilisch-Neumann A, Daniel EA, et al. Receptor tyrosine kinase inhibition by regorafenib/sorafenib inhibits growth and invasion of meningioma cells. Eur J Cancer 2017;73:9-21. [Crossref] [PubMed]
- Pachow D, Andrae N, Kliese N, et al. mTORC1 inhibitors suppress meningioma growth in mouse models. Clin Cancer Res 2013;19:1180-9. [Crossref] [PubMed]
- Nigim F, Esaki S-i, Hood M, et al. A new patient-derived orthotopic malignant meningioma model treated with oncolytic herpes simplex virus. Neuro Oncol 2016;18:1278-87. [Crossref] [PubMed]
- Ragel BT, Elam IL, Gillespie DL, et al. A novel model of intracranial meningioma in mice using luciferase-expressing meningioma cells. Laboratory investigation. J Neurosurg 2008;108:304-10. [Crossref] [PubMed]
- McCutcheon IE, Friend KE, Gerdes TM, et al. Intracranial injection of human meningioma cells in athymic mice: an orthotopic model for meningioma growth. J Neurosurg 2000;92:306-14. [Crossref] [PubMed]
- Salhia B, Rutka JT, Lingwood C, et al. The treatment of malignant meningioma with verotoxin. Neoplasia 2002;4:304-11. [Crossref] [PubMed]
- Baia GS, Dinca EB, Ozawa T, et al. An orthotopic skull base model of malignant meningioma. Brain Pathol 2008;18:172-9. [Crossref] [PubMed]
- Chow HY, Dong B, Duron SG, et al. Group I Paks as therapeutic targets in NF2-deficient meningioma. Oncotarget 2015;6:1981-94. [Crossref] [PubMed]
- Burns SS, Akhmametyeva EM, Oblinger JL, et al. Histone deacetylase inhibitor AR-42 differentially affects cell-cycle transit in meningeal and meningioma cells, potently inhibiting NF2-deficient meningioma growth. Cancer Res 2013;73:792-803. [Crossref] [PubMed]
- Gogineni VR, Nalla AK, Gupta R, et al. Chk2-mediated G2/M cell cycle arrest maintains radiation resistance in malignant meningioma cells. Cancer Lett 2011;313:64-75. [Crossref] [PubMed]
- Gogineni VR, Nalla AK, Gupta R, et al. alpha3beta1 integrin promotes radiation-induced migration of meningioma cells. Int J Oncol 2011;38:1615-24. [PubMed]
- Gupta R, Nalla AK, Gogineni VR, et al. uPAR/cathepsin B overexpression reverse angiogenesis by rescuing FAK phosphorylation in uPAR/cathepsin B down regulated meningioma. PLoS One 2011;6:e17123. [Crossref] [PubMed]
- van Furth WR, Laughlin S, Taylor MD, et al. Imaging of murine brain tumors using a 1.5 Tesla clinical MRI system. Can J Neurol Sci 2003;30:326-32. [Crossref] [PubMed]
- Burns SS, Chang LS. Generation of Noninvasive, Quantifiable, Orthotopic Animal Models for NF2-Associated Schwannoma and Meningioma. In: Sokolowski B, editor. Auditory and Vestibular Research: Methods and Protocols. New York, NY: Springer New York; 2016:59-72.
- Iwami K, Natsume A, Ohno M, et al. Adoptive transfer of genetically modified Wilms’ tumor 1-specific T cells in a novel malignant skull base meningioma model. Neuro Oncol 2013;15:747-58. [Crossref] [PubMed]


