Management of hepatocellular in the United States
Introduction
Hepatocellular carcinoma (HCC) is a leading cause of cancer burden globally. In several countries in Asia, HCC is the most common cause of cancer deaths (1). In Europe, the number of new HCC cases has increased dramatically over the past two decades (2). Incident HCC cases have almost doubled during the same period in the United States, and are forecasted to continue to rise over the next 15 years (3).
The majority of patients with HCC have underlying chronic liver dysfunction and/or liver cirrhosis (4). The cause of liver dysfunction is dependent on incident geographic region, and is mainly related to chronic hepatitis B or C, alcohol abuse, or non-alcoholic fatty liver disease (5). The concomitant liver dysfunction and tumor burden further complicate the HCC treatment paradigm influencing decision-making and ultimately patient prognosis. The challenge lies in delivering curative and life-prolonging treatments without negatively impacting the underlying liver dysfunction thus causing hepatic decompensation. A multidisciplinary treatment approach encompassing the specialties of surgery, oncology, hepatology, radiology, and palliative care is needed to bridge the gap between liver function and tumor biology to ensure appropriate HCC treatment decisions. Our group and others have demonstrated the benefits of a multidisciplinary approach to HCC care in terms of improved patient outcomes (6-8).
The Barcelona Clinic Liver Cancer (BCLC) algorithm is an externally validated staging system providing a framework for the management of HCC, and has been adopted by the European Association for Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) (9-11). In the BCLC staging system, treatment is based on tumor burden, liver function, and Eastern Cooperative Oncology Group (ECOG) patient performance status (PS) (Figure 1). Patients with preserved liver function and low tumor burden (BCLC 0 and A) are amenable to curative treatments, whereas those with poor liver function (Child Pugh C) and/or prohibitive performance status (PS >2) are limited to supportive care only (BCLC D). In the middle of these two extremes of the HCC staging and treatment spectrum is a group of patients with both large tumor burden and preserved liver function for whom palliative treatment options are appropriate.
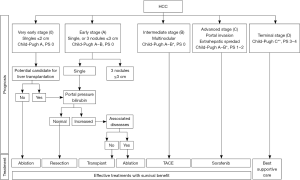
The aim of this review is to discuss current HCC treatment paradigms according to the BCLC staging system and to highlight emerging novel therapies.
BCLC 0 and A
BCLC A, or early stage HCC, includes patients with low tumor burden––specifically, a solitary lesion or up to three nodules smaller than 3 cm––with preserved liver function and performance status. Patients with a single nodule smaller than 2 cm constitute very early stage HCC or BCLC 0.
Treatment for patients with very early and early stage HCC is curative, and comprises tumor ablation, liver resection, or orthotopic liver transplant. To date, however, the data guiding the choice of treatment is inconclusive and is largely dependent on patient comorbidities and institutional preference or resources.
Liver resection and radiofrequency ablation (RFA) for early stage HCC have been compared in three randomized clinical trials, each reporting dissimilar results. Two trials demonstrated similar 3-year overall survival (OS) and recurrence-free survival (RFS) between liver resection and RFA (OS: 73–75% vs. 67–69%; RFS: 61–69% vs. 50–60%, P value not significant for both comparisons) (12,13), whereas the third showed improved overall and recurrence-free survival at five years in the resection group (OS: 92% vs. 70%, P<0.01; RFS: 61% vs. 46%, P=0.02) (14). The varying results may be explained in part by differences in tumor size across the three trials, since efficacy following radiofrequency ablation correlates to tumor size (15).
Comparative studies according to tumor size in patients with early stage HCC failed however to provide consistent evidence favoring RFA or liver resection based on size. In patients with a single HCC nodule smaller than 2 cm, several observational studies demonstrated similar overall survival between RFA and liver resection (16-18). For HCC lesions greater than 2 cm (and up to 5 cm), liver resection was associated with similar overall survival in some studies, and better overall survival in others, compared to RFA (13,14,19-21). Patient and tumor characteristics in these observational studies (and a subgroup analysis in two randomized trials) were dissimilar between the two treatment groups; patients undergoing RFA were more likely to be older, a more advanced Child-Pugh class, and have lower platelet counts.
Given the limitations of RFA to achieve complete tumor necrosis in large lesions, the addition of transarterial chemoembolization (TACE) to RFA was proposed as means to improve treatment response. TACE involves injecting a chemotherapeutic agent into the arterial branches feeding the tumor followed by selective embolization of these vessels. The combination therapy offers several potential advantages over RFA alone including a larger ablation zone secondary to reduced heat loss following embolization, more precise assessment of tumor margins, and better control of satellite lesions. A meta-analysis of seven randomized clinical trials demonstrated improved overall survival at three years with combination RFA and TACE compared to RFA alone [odds ratio (OR) =2.27; 95% CI: 1.57–3.27] without an increase in major complications (OR =1.26; 95% CI: 0.33–4.77) (22). When examined according to tumor size, the overall survival benefit following combination therapy was evident in patients with HCC nodules larger than 3 cm, but there was no difference in survival for patients with lesions smaller than 3 cm (22). Improved survival with RFA and TACE did not demonstrate a survival benefit over liver resection, however. Both 5-year overall survival and 5-year recurrence-free survival were worse following TACE and RFA compared to liver resection in a recent randomized clinical trial (OS: 46% vs. 62%, P 0.01; RFS: 36% vs. 48%, P=0.03) (23). In addition, multiple retrospective studies showed equivalent overall survival between the two treatments for HCC lesions smaller than 5 cm; recurrence-free survival was either equivalent or better following resection in these studies (24-27).
Microwave ablation, an alternative ablation system, is a heat-based ablation technique that generates higher intra-tumoral temperatures more rapidly compared RFA systems. Microwave ablation is less susceptible to heat sink effects secondary to tumor abutment of large vessels and produces larger ablation areas compared to RFA (28). The evidence to date indicates that microwave ablation is at least equivalent to RFA for the treatment of very early and early stage HCC, but there is a paucity of studies with direct comparison between the two ablative technologies (29-31).
Although head-to-head comparisons between ablation and liver resection for the treatment of very early and early stage HCC do not strongly favor one technique over the other, the probability that a patient is truly eligible for both treatments is unlikely in clinical practice. For instance, liver resection is generally contraindicated in patients with portal hypertension, elevated bilirubin, severe comorbidities or advanced age, as well as in cases where a prohibitively extensive parenchymal resection would be required due to a small functional liver remnant. On other hand, ablation of subcapsular tumors or tumors near the gallbladder, the diaphragm or vital vessels/biliary branches confers a high risk to the patient. Accordingly, the selection of treatment, liver resection or ablation, is more often driven by factors beyond those addressed in comparative studies (32).
Orthotopic liver transplant is the treatment of choice for patients with HCC. Liver transplantation removes the tumor and eliminates the liver dysfunction as well as the predisposition to tumor recurrence. Comparative evaluations from observational studies between liver transplant and other treatment modalities for HCC have consistently demonstrated better outcomes following liver transplant. In a meta-analysis of 62 studies, liver transplant was associated with improved overall survival (OR =1.77; 95% CI: 1.45–2.16), and recurrence-free survival (OR =5.58; 95% CI: 4.12–7.55), as well as lower recurrence (OR =0.2; 95% CI: 0.15–0.28) compared to liver resection (33). In patients with early stage HCC and Child-Pugh class A cirrhosis, overall survival was comparable between both liver transplant and resection, albeit the recurrence rate remained higher following liver resection. In this subset group, liver resection is possibly more cost-effective than liver transplant (34).
Liver transplants can be deceased donor or living donor transplants. In the United States and Europe, deceased donor liver transplants (DDLT) are performed routinely, whereas over 90% of liver transplants in Asia are living donor liver transplants (LDLT) (35). In a meta-analysis that included 633 LDLT and 1232 DDLT accrued from 12 observational studies, recurrence-free survival was worse in LDLT compared to DDLT (HR =1.59; 95% CI: 1.02–2.49), but overall survival was comparable (HR =0.97; 95% CI: 0.73–1.27) (36).
The main limitation of liver transplant is shortage of organ donors. Thus, liver transplant has been prioritized to patients expected to gain the most benefit from the procedure. At present, most treating institutions adopted the Milan criteria for allocation of cadaveric livers for transplantation. Patients within the Milan criteria and eligible for donor allocation meet the following criteria: one tumor less than 5 cm or up to three nodules less than 3 cm each without extrahepatic metastasis or macrovascular tumor invasion (37). Five-year overall survival following liver transplant for patients within Milan criteria exceeds 65% which approximates the survival of patients undergoing liver transplant for non-tumor indications (38). It is this comparability in overall survival following liver transplant between HCC patients within Milan criteria and patients without cancer that prompted rapid adoption of Milan criteria and justified donor liver allocation to HCC patients.
The Milan criteria were first described more than two decades ago and have remained the standard criteria for cadaveric liver allocation. Several groups, however, have argued that the criteria are too restrictive and may exclude patients, which would have otherwise benefited from transplantation. The group at the University of California San Francisco (UCSF) proposed expanding the Milan criteria to include one nodule smaller than 6.5 cm or as many as 3 nodules smaller than 4.5 cm and a total tumor diameter size up to 8 cm (39). Several other expansion criteria proposals have been described, none however has been accepted as standard criteria in lieu of Milan for selecting liver transplant candidates with HCC (40). The increased likelihood of underestimating tumor size for lesions beyond Milan criteria (41,42), the large overlap with Milan criteria leading to only modest increase in eligible patients (5% to 10% increase with the UCSF criteria) (43), and the need for prospective validation studies with large enough sample sizes to compare outcomes of patients within Milan criteria to those beyond Milan criteria constitute some of several reasons that have hampered adoption of expanded criteria for liver transplant (40,44).
Basing transplant selection criteria on tumor size and number of nodules highlights the importance of these factors in predicting tumor recurrence post-transplant and patient survival. Following liver transplantation, overall survival can be predicted according to a predictive survival model developed by the Metroticket Investigator Study Group which incorporates different combinations of tumor size and nodule number, as well as the presence or absence of microvascular invasion (45). Large tumor size and increased nodule number are associated with increased risk of microvascular invasion, poor differentiation, and microsatellite lesions, all of which are surrogates to tumor aggressiveness (38). It has been argued, however, that tumor size and number of lesions display a one-time static snapshot of a patient’s tumor. “Dynamic” tumor characteristic may reflect tumor biology better and potentially predict recurrence more accurately. Accordingly, tumor response to TACE and progression of alfa-fetoprotein (AFP) levels over time have been proposed to supplement tumor size and nodule number. Complete response, as well as partial response, following TACE were associated with improved survival post-transplant compared to patients that did not response to TACE (46,47). Also, an increase of pre-transplant AFP level beyond 15 ng/mL per month predicted worse 5-year survival compared to patients in whom AFP progression was absent or less than 15 ng/mL/month (48). Future studies validating the predictive power of responsiveness to TACE and AFP progression may motivate incorporating these markers into the selection criteria in HCC patients for liver transplant.
Tumor downstaging to meet Milan criteria is an ancillary method used to maximize the number of patients with HCC to receive a liver transplant. Locoregional therapies including transarterial chemoembolization (TACE) are most commonly used in downstaging treatment. Other treatment modalities including ablation, resection, and radioembolization have been employed as well (40). Following successful downstaging therapy to within the Milan or institutionally adopted criteria, patients typically wait time 3 to 6 months prior to undergoing transplantation (49). The wait time has been adopted recently as means to exclude patients with aggressive tumor biology. In a meta-analysis of 13 studies, nearly half of patients initiated on downstaging therapy were successfully downstaged to within Milan criteria (50). Overall survival post-transplant was widely variable across studies, and recurrence was 16%, which is considerably higher compared to patients originally within Milan criteria (less than 5%) (45,50). At present, downstaging is unlikely to receive robust endorsement due to inconsistent entry criteria, lack of validation studies, as well as heterogeneity in downstaging protocols and outcomes assessment standards.
BCLC B
Patients with BCLC B, or intermediate stage HCC, have a large tumor burden not amenable to curative treatment, but no evidence of spread extrahepatically or within major vascular structures. These patients also have preserved liver function and limited cancer-related symptoms impacting performance status. Locoregional therapies including TACE and transarterial radioembolization remain the mainstay of treatment for BCLC B patients.
The treatment of HCC with TACE has been largely driven by two randomized clinical trials that demonstrated improved overall survival following TACE (compared to supportive care) in patients that are not amenable to curative options (51,52). In the first trial, Lo et al. demonstrated a survival benefit in patients randomized to TACE using an emulsion of cisplatin mixed in Lipiodol and gelatin sponge embolic particles compared to patients randomized to best supportive care (HR =0.50; 95% CI: 0.3–0.81) (52). In the second trial, Llovet et al. reported improved survival in patients undergoing TACE using a doxorubicin-Lipiodol emulsion with gelatin sponge embolic particles compared to supportive care (HR =0.47; 95% CI: 0.25–0.91) (51).
The trial findings reported by Lo et al. and Llovet et al. have been challenged by a Cochrane review which concluded there is not enough evidence to support TACE for patients with unresectable HCC (53). The discrepancy in the results underscores the large variability in TACE administration protocols among institutions, the number of TACE treatments administered, and the timing of follow-up imaging. Nevertheless, TACE has been adopted as the standard of care for the treatment of intermediate stage HCC at most institutions, and it is unlikely that any further clinical trials comparing TACE to supportive care would be pursued.
TACE is a catheter-based intra-arterial procedure that exploits the predominant hepatic arterial supply to HCC lesions. In a conventional TACE procedure, a chemotherapeutic agent, usually doxorubicin or cisplatin mixed with Lipiodol which increases exposure of the tumor to the drug, is delivered into the hepatic arterial branches supplying the HCC lesion, then followed by selective embolization of these branches with embolic particles (54). The resultant cytotoxicity and tissue ischemia induce tumor necrosis. A more recently introduced Lipiodol-free delivery system that uses doxorubicin loaded drug-eluting beads, DEB-TACE, delivering chemotherapy to the tumor in a more controlled and sustained fashion. Compared to conventional TACE, DEB-TACE is better tolerated largely due to lower systemic concentrations of doxorubicin. There has been no difference, however, in response rates or tumor progression and survival between conventional TACE and DEB-TACE according to two randomized controlled trials (55,56).
Not all patients with intermediate stage HCC benefit equally from TACE. Optimal candidates are patients with solitary or limited multifocal disease and relatively well-preserved liver function (9,57). In those, the median survival following TACE may be as high as 40 months and serious complications such as post-embolization syndrome (abdominal pain, fever, and nausea) and liver failure occur in less than 5 percent of cases (58). On the other hand, patients with extensive disease (>10 cm), poor residual liver function, impaired portal blood flow, and/or untreated high-risk varices are likely to suffer serious adverse events such that TACE becomes contraindicated (9,57).
Effective treatment of HCC typically necessitates delivering more than one cycle of TACE (59). Multiple treatments may be given either at fixed time intervals, also known as scheduled TACE, or on-demand according to treatment response after each TACE cycle. It is clear, however, that an aggressive TACE schedule increases the incidence of complications (60,61). Treatment of TACE is usually repeated unless no substantial tumor response is demonstrated after two cycles of TACE, significant progression ensues including vascular invasion, extrahepatic spread, or untreatable growth/new lesion, or deterioration in liver function or performance status renders retreatment unsafe (62). The decision to discontinue TACE could be further guided by scoring systems, such as the Assessment for Retreatment with TACE (ART) score, which incorporates radiologic response, Child-Pugh score, and AST level to identify patients that are less likely to benefit from additional TACE procedures (63).
Radioembolization, or TARE, is another locoregional treatment option for patients with intermediate stage HCC. In this procedure, microspheres carrying yttrium-90 (90Y), a high-energy radiation emitter with a short half-life (2.7 days) and shallow tissue penetrance, are injected selectively into the arterial branches supplying the HCC lesion. The anti-tumoral effects are mediated by microembolization of the tumor microvasculature with high-energy radiation emission (64).
TARE is equally efficacious to TACE in patients with intermediate stage HCC according to several observational studies. In a retrospective review of 103 patients with BCLC B, tumor response by EASL criteria was similar following TARE and TACE (71% vs. 66%, P=0.66) (65). Time-to-progression was longer following TARE (13.3 months vs. 9.4 months, P=0.05); however, this benefit did not translate to improvement in overall survival (median survival: 17.2 vs. 17.5 months, P=0.42). Another retrospective review that compared TARE and TACE demonstrated a slight advantage in overall survival following TARE in patients without portal vein thrombus or extrahepatic metastasis (median survival: 16 months vs. 12 months, P<0.05) (66). To date, there has not been any randomized prospective head-to-head comparisons between both treatments, and it is unlikely that there would be any, given the prohibitive sample sizes required to adequately power such a study (67).
Beyond treatment efficacy, there are several advantages to treatment with TARE compared to TACE. TARE is generally performed in an outpatient setting, whereas TACE requires hospitalization (65,66). Unlike TACE, repeated treatment with TARE is seldom needed to achieve response. In addition, TARE is typically better tolerated. The most common side effect following TARE is transient fatigue, whereas serious adverse events (grade 3 and 4 toxicities) occur in less that 5% of cases. On the other hand, abdominal pain, nausea, emesis, and fever––the post-embolization syndrome––are more common after TACE. Finally, TARE, unlike TACE, can be performed in patients with portal vein thrombosis.
TARE, however, is costlier than TACE. Based on Medicare reimbursement in the United States, one TACE session including an overnight hospitalization costs $17,000; in contrast, TARE costs $31,000 ($48,000 in case an intervention is performed in both liver lobes) (68). Nevertheless, the cost-effectiveness of TARE in comparison to TACE as the standard of care for BCLC B patients is yet to be defined. Additional costs incurred secondary to repeated treatment cycles and hospitalization for complications as well as valid outcome estimates have not been properly addressed in previously published cost-effectiveness studies.
BCLC C
BCLC C, or advanced stage HCC, comprises patients with tumor extension into the hepatic vasculature, usually the portal or hepatic veins, patients with spread beyond the liver (including extrahepatic nodal metastasis), and/or patients with cancer-related symptoms (performance status 1 or 2). This classification emanated from the landmark BCLC study in 1999, which showed portal invasion, metastasis, and constitutional symptoms (or performance status) were independent predictors of worse prognosis in patients with unresectable HCC and preserved liver function (69). Within the BCLC C group, there remains marked variability in patient prognosis and several recent studies concluded that further stratification by vascular invasion and distant spread is warranted (70,71).
The standard of care for patients with advanced stage HCC, at present, is treatment with the systemic agent, sorafenib. Sorafenib, an oral multikinase inhibitor with effects on cell proliferation, angiogenesis, and cell apoptosis (72), was approved for the treatment of advanced HCC in 2008 after demonstrating a survival benefit compared to supportive care in two landmark phase III randomized clinical trials: the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial and the Asia-Pacific trial. In the SHARP trial, a total of 602 patients with advanced stage HCC accrued from over 120 centers in Europe, the Americas, and Australia were randomized to receive sorafenib or placebo. There was a longer time to radiologic progression in the sorafenib group (5.5 vs. 2.9 months, P<0.01) and nearly a 3-month improvement in overall survival compared to placebo (median overall survival: 7.9 vs. 10.7 months, P<0.01) (73). The companion Asia-Pacific randomized controlled phase III trial demonstrated the efficacy of sorafenib in a predominantly Asian cohort of HCC patients. Similar findings to the SHARP trial were shown including doubling of the time to progression (2.8 vs. 1.4 months, P<0.01) and over a 2-month improvement in overall survival (6.5 months vs. 4.2 months, P<0.01) in the sorafenib group (74).
The addition of sorafenib to the treatment of HCC represents a breakthrough in the management of patients with advanced HCC who were, until 2008, offered predominantly supportive care. Sorafenib, however, has several limitations. First, sorafenib is poorly tolerated such that 20 to 38 percent of patients discontinue the drug due to drug-related adverse events (73,74). The most common serious side effects (toxicity grades 3 and 4) are hand-foot skin reaction and diarrhea. Interestingly, having adverse events correlates with better outcome (75). Second, primary resistance is a significant issue which has reflected in disease control rates are rarely exceeding 50% (73,74). Secondary resistance usually develops after several weeks of therapy initiation, which may explain the short time to progression intervals in the SHARP and Asia-Pacific trials. Last, there is very little evidence that sorafenib impacts survival in patients with Child-Pugh classes B and C (76).
Following sorafenib, several agents have been evaluated in phase III trials for the treatment of HCC (Table 1). In the first-line setting, none of the trials completed to date demonstrated a survival benefit (or non-inferiority) to sorafenib for advanced stage HCC (77-80). Similarly, multiple agents proved non-efficacious in comparison to placebo in second-line trials (81-84). Failure of these trials has been attributed to various reasons including suboptimal evaluation of drug-induced liver toxicity, poor phase II study design, questionable value of time to progression and objective response rate as surrogate endpoints for survival, and absence of biomarker analysis (86,87). The latter reason is particularly important as the overwhelming majority of trials failed to acquire tumor tissue, and as a result, an evaluation of drug efficacy in molecularly selected patient subgroups was not possible.
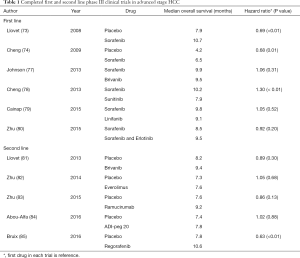
Full table
Recently a second line treatment of advanced HCC in patients failing first line sorafenib therapy was reported. The Study of Regorafenib After Sorafenib in Patients with Hepatocellular Carcinoma (RESORCE) trial demonstrated that regorafenib, an oral multikinase inhibitor with a similar target profile to sorafenib, was shown to extend survival compared to placebo (median survival: 10.6 months vs. 7.8 months, P<0.01) in patients with intermediate and advanced HCC that progressed on first-line sorafenib (85). With these findings, regorafenib would be the only second-line agent for the treatment of HCC to demonstrate efficacy.
The search for agents more effective and better tolerated than sorafenib for the treatment of advanced HCC continues to be very active. The majority of ongoing clinical trials investigating these agents can be broadly classified into two types: patient enrichment trials and immune checkpoint inhibitor trials. Table 2 details several open patient enrichment trials. For instance, the JET-HCC trial (NCT02029157) is a phase III trial evaluating tivantinib for second-line treatment of advanced stage HCC in patients with MET-high tumors. It follows a placebo-controlled phase II trial in which the subset of patients with MET-high tumors demonstrated improved overall survival after treatment with tivantinib (88). Similarly, ramucirumab is being investigated in HCC patients with elevated AFP (400 ng/mL) following favorable outcomes in a subgroup of patients with increased levels of AFP levels in a trial of ramucirumab as a second-line therapy (83). The second type of trials in advanced stage HCC concerns immune check-point inhibitors such as inhibitors of PD-1 and CTLA-4. In early phase trials, nivolumab, a PD-1 inhibitor, and tremelimumab, a CTLA-4 inhibitor, showed promising response rates in patients with advanced HCC (89,90). Confirmatory phase III trials are currently underway (Table 3).

Full table
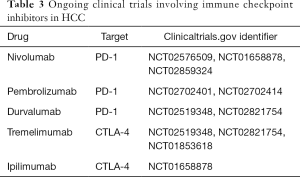
Full table
BCLC D
HCC patients with poor liver function (Child-Pugh class C) and/or prohibitive ECOG performance status (PS >2) are classified BCLC D, or terminal stage HCC, irrespective of tumor burden. The prognosis of this patient group is weeks to few months, and they are managed with best supportive care. Notably, BCLC D patients secondary to impaired liver function in whom HCC is within transplant criteria may be considered for liver transplant.
Conclusions
In conclusion, HCC is a significant and increasing cause of cancer burden globally and in the western world. Patient prognosis remains dismal and novel treatment options, particularly for patients with advanced HCC, are lagging owing to our poor understanding of the molecular drivers of HCC. At present, this is an active area of research that holds promise to uncover implicating carcinogenic pathways and identify actionable genetic and molecular aberrations that can be translated into targeted therapies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, et al. The Global Burden of Cancer 2013. JAMA Oncol 2015;1:505-27. [Crossref] [PubMed]
- International Agency for Research on Cancer. Liver Cancer: Estimated Incidence, Mortality and Prevalence Worldwide in 2012 [Internet]. GLOBOCAN. 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Petrick JL, Kelly SP, Altekruse SF, et al. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol 2016;34:1787-94. [Crossref] [PubMed]
- Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis 2010;30:3-16. [Crossref] [PubMed]
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264-1273.e1. [Crossref] [PubMed]
- Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol 2014;21:1287-95. [Crossref] [PubMed]
- Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford) 2008;10:405-11. [Crossref] [PubMed]
- Zhang J, Mavros MN, Cosgrove D, et al. Impact of a single-day multidisciplinary clinic on the management of patients with liver tumours. Curr Oncol 2013;20:e123-31. [Crossref] [PubMed]
- European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Bruix J, Reig M, Sherman M. Evidence-Based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016;150:835-53. [Crossref] [PubMed]
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012;57:794-802. [Crossref] [PubMed]
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321-8. [Crossref] [PubMed]
- Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010;252:903-12. [Crossref] [PubMed]
- Lu DS, Yu NC, Raman SS, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology 2005;234:954-60. [Crossref] [PubMed]
- Takayama T, Makuuchi M, Hasegawa K. Single HCC smaller than 2 cm: surgery or ablation?: surgeon's perspective. J Hepatobiliary Pancreat Sci 2010;17:422-4. [Crossref] [PubMed]
- Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013;31:426-32. [Crossref] [PubMed]
- Wang JH, Wang CC, Hung CH, et al. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol 2012;56:412-8. [Crossref] [PubMed]
- Ueno S, Sakoda M, Kubo F, et al. Surgical resection versus radiofrequency ablation for small hepatocellular carcinomas within the Milan criteria. J Hepatobiliary Pancreat Surg 2009;16:359-66. [Crossref] [PubMed]
- Hiraoka A, Horiike N, Yamashita Y, et al. Efficacy of radiofrequency ablation therapy compared to surgical resection in 164 patients in Japan with single hepatocellular carcinoma smaller than 3 cm, along with report of complications. Hepatogastroenterology 2009;55:2171-4. [PubMed]
- Lupo L, Panzera P, Giannelli G, et al. Single hepatocellular carcinoma ranging from 3 to 5 cm: radiofrequency ablation or resection? HPB (Oxford) 2007;9:429-34. [Crossref] [PubMed]
- Lu Z, Wen F, Guo Q, et al. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol 2013;25:187-94. [Crossref] [PubMed]
- Liu H, Wang ZG, Fu SY, et al. Randomized clinical trial of chemoembolization plus radiofrequency ablation versus partial hepatectomy for hepatocellular carcinoma within the Milan criteria. Br J Surg 2016;103:348-56. [Crossref] [PubMed]
- Takuma Y, Takabatake H, Morimoto YA, et al. Comparison of combined transcatheter arterial chemoembolization and radiofrequency ablation with surgical resection by using propensity score matching in patients with hepatocellular carcinoma within Milan criteria. Radiology 2013;269:927-37. [Crossref] [PubMed]
- Kagawa T, Koizumi J, Kojima S, et al. Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma: comparison with surgical resection. Cancer 2010;116:3638-44. [Crossref] [PubMed]
- Yamakado K, Nakatsuka A, Takaki H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology 2008;247:260-6. [Crossref] [PubMed]
- Kim JW, Shin SS, Kim JK, et al. Radiofrequency ablation combined with transcatheter arterial chemoembolization for the treatment of single hepatocellular carcinoma of 2 to 5 cm in diameter: comparison with surgical resection. Korean J Radiol 2013;14:626-35. [Crossref] [PubMed]
- Wells SA, Hinshaw JL, Lubner MG, et al. Liver ablation: best practice. Radiol Clin North Am 2015;53:933-71. [Crossref] [PubMed]
- Vogl TJ, Farshid P, Naguib NN, et al. Ablation therapy of hepatocellular carcinoma: a comparative study between radiofrequency and microwave ablation. Abdom Imaging 2015;40:1829-37. [Crossref] [PubMed]
- Abdelaziz A, Elbaz T, Shousha HI, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc 2014;28:3429-34. [Crossref] [PubMed]
- Ding J, Jing X, Liu J, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol 2013;82:1379-84. [Crossref] [PubMed]
- Cucchetti A, Piscaglia F, Cescon M, et al. Systematic review of surgical resection vs radiofrequency ablation for hepatocellular carcinoma. World J Gastroenterol 2013;19:4106-18. [Crossref] [PubMed]
- Zheng Z, Liang W, Milgrom DP, et al. Liver transplantation versus liver resection in the treatment of hepatocellular carcinoma: a meta-analysis of observational studies. Transplantation 2014;97:227-34. [Crossref] [PubMed]
- Lim KC, Wang VW, Siddiqui FJ, et al. Cost-effectiveness analysis of liver resection versus transplantation for early hepatocellular carcinoma within the Milan criteria. Hepatology 2015;61:227-37. [Crossref] [PubMed]
- Shukla A, Vadeyar H, Rela M, et al. Liver transplantation: East versus West. J Clin Exp Hepatol 2013;3:243-53. [Crossref] [PubMed]
- Grant RC, Sandhu L, Dixon PR, et al. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transplant 2013;27:140-7. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Mazzaferro V, Bhoori S, Sposito C, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl 2011;17:S44-57. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [Crossref] [PubMed]
- Silva MF, Sherman M. Criteria for liver transplantation for HCC: what should the limits be? J Hepatol 2011;55:1137-47. [Crossref] [PubMed]
- Decaens T, Roudot-Thoraval F, Hadni-Bresson S, et al. Impact of UCSF criteria according to pre- and post-OLT tumor features: analysis of 479 patients listed for HCC with a short waiting time. Liver Transpl 2006;12:1761-9. [Crossref] [PubMed]
- Silva M, Moya A, Berenguer M, et al. Expanded criteria for liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Liver Transpl 2008;14:1449-60. [Crossref] [PubMed]
- Prasad KR, Young RS, Burra P, et al. Summary of candidate selection and expanded criteria for liver transplantation for hepatocellular carcinoma: a review and consensus statement. Liver Transpl 2011;17 Suppl 2:S81-9. [Crossref] [PubMed]
- Yopp AC, Marrero JA, Singal AG. Expansion of Criteria for Liver Transplantation in Hepatocellular Carcinoma: Better Patient Selection or a Slippery Slope? Ann Surg Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [Crossref] [PubMed]
- Otto G, Herber S, Heise M, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl 2006;12:1260-7. [Crossref] [PubMed]
- Millonig G, Graziadei IW, Freund MC, et al. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl 2007;13:272-9. [Crossref] [PubMed]
- Vibert E, Azoulay D, Hoti E, et al. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant 2010;10:129-37. [Crossref] [PubMed]
- Pomfret EA, Washburn K, Wald C, et al. Report of a National conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl 2010;16:262-78. [Crossref] [PubMed]
- Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transpl 2015;21:1142-52. [Crossref] [PubMed]
- Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. [Crossref] [PubMed]
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164-71. [Crossref] [PubMed]
- Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Available online: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD004787.pub2/abstract
- de Baere T, Arai Y, Lencioni R, et al. Treatment of Liver Tumors with Lipiodol TACE: Technical Recommendations from Experts Opinion. Cardiovasc Intervent Radiol 2016;39:334-43. [Crossref] [PubMed]
- Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52. [Crossref] [PubMed]
- Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer 2014;111:255-64. [Crossref] [PubMed]
- Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 2011;37:212-20. [Crossref] [PubMed]
- Takayasu K, Arii S, Kudo M, et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol 2012;56:886-92. [Crossref] [PubMed]
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429-42. [Crossref] [PubMed]
- Ernst O, Sergent G, Mizrahi D, et al. Treatment of hepatocellular carcinoma by transcatheter arterial chemoembolization: comparison of planned periodic chemoembolization and chemoembolization based on tumor response. AJR Am J Roentgenol 1999;172:59-64. [Crossref] [PubMed]
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002;224:47-54. [Crossref] [PubMed]
- Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol 2015;62:1187-95. [Crossref] [PubMed]
- Hucke F, Sieghart W, Pinter M, et al. The ART-strategy: sequential assessment of the ART score predicts outcome of patients with hepatocellular carcinoma re-treated with TACE. J Hepatol 2014;60:118-26. [Crossref] [PubMed]
- Salem R, Mazzaferro V, Sangro B. Yttrium 90 radioembolization for the treatment of hepatocellular carcinoma: biological lessons, current challenges, and clinical perspectives. Hepatology 2013;58:2188-97. [Crossref] [PubMed]
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer Time-to-Progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011;140:497-U205. [Crossref] [PubMed]
- Carr BI, Kondragunta V, Buch SC, et al. Therapeutic equivalence in survival for hepatic arterial chemoembolization and Yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer 2010;116:1305-14. [Crossref] [PubMed]
- Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138:52-64. [Crossref] [PubMed]
- Rostambeigi N, Dekarske AS, Austin EE, et al. Cost effectiveness of radioembolization compared with conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Vasc Interv Radiol 2014;25:1075-84. [Crossref] [PubMed]
- Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: Rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29:62-7. [Crossref] [PubMed]
- Sinn DH, Cho JY, Gwak GY, et al. Different survival of Barcelona clinic liver cancer stage C hepatocellular carcinoma patients by the extent of portal vein invasion and the type of extrahepatic spread. PLoS One 2015;10:e0124434. [Crossref] [PubMed]
- Mokdad AA, Singal AG, Marrero JA, et al. Vascular invasion and metastasis is predictive of outcome in Barcelona clinic liver cancer stage C hepatocellular carcinoma. J Natl Compr Canc Netw 2017;15:197-204. [PubMed]
- Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 2006;5:835-44. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Reig M, Torres F, Rodriguez-Lope C, et al. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol 2014;61:318-24. [Crossref] [PubMed]
- Marrero JA, Lencioni R, Ye SL, et al. Final analysis of GIDEON (Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma [HCC] and of Its Treatment with Sorafenib [Sor]) in >3000 Sor-treated patients (pts): Clinical findings in pts with liver dysfunction. J Clin Oncol 2013;31:abstr 4126.
- Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol 2013;31:3517-24. [Crossref] [PubMed]
- Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol 2013;31:4067-75. [Crossref] [PubMed]
- Cainap C, Qin S, Huang WT, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol 2015;33:172-9. [Crossref] [PubMed]
- Zhu AX, Rosmorduc O, Evans TR, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2015;33:559-66. [Crossref] [PubMed]
- Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol 2013;31:3509-16. [Crossref] [PubMed]
- Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 2014;312:57-67. [Crossref] [PubMed]
- Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015;16:859-70. [Crossref] [PubMed]
- Abou-Alfa GK, Qin S, Ryoo BY, et al. Phase III randomized study of second line ADI-peg 20 (A) plus best supportive care versus placebo (P) plus best supportive care in patients (pts) with advanced hepatocellular carcinoma (HCC). J Clin Oncol 2016;34:abstr 4017.
- Bruix J, Qin S, Merle P, et al. Phase III randomized study of second line ADI-peg 20 (A) plus best supportive care versus placebo (P) plus best supportive care in patients (pts) with advanced hepatocellular carcinoma (HCC). J Clin Oncol 2016;34:abstr 4017.
- Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res 2014;20:2072-9. [Crossref] [PubMed]
- Mokdad AA, Singal AG, Yopp AC. Advances in Local and Systemic Therapies for Hepatocellular Cancer. Curr Oncol Rep 2016;18:9. [Crossref] [PubMed]
- Santoro A, Rimassa L, Borbath I, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol 2013;14:55-63. [Crossref] [PubMed]
- El-Khoueiry AB, Melero I, Crocenzi TS, et al. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209-040. J Clin Oncol 2015;33:abstr LBA101.
- Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81-8. [Crossref] [PubMed]


