Evaluation of miR-22 and miR-20a as diagnostic biomarkers for gastric cancer
Introduction
Gastric cancer (GC) is the second reason for non-accidental death in Iran. Racial differences, different diets as well as various geographical distribution have led to various cancer prevalence in different regions of the country (1,2). Throughout the world GC is the fourth common malignancy and is the second cause of mortality due to cancer (3). The considerable point is that the incidence of GC is higher than global average among Iranian males (1). Some risk factors involved in developing GC include gender (more among males comparing with females), age (over 50), geographical distribution, infections due to helicobacter pylori, diet, smoking, obesity, and blood group A (4).
Diagnosing GC in its earlier stages is followed by more successful treatments. Unfortunately, diagnosing and treating GC is complicated due to lack of specific diagnostic symptoms in its early stages, limitation and inefficiency of existing diagnostic approaches such as endoscopy and imaging methods (5), ineffective surgery or treatment with cytotoxic cells in their advanced stages, and lack of suitable biomarker (6). Therefore, for better diagnosis and successful treatment, molecular markers are required in different phases of the disease (7). Currently, there are a few biomarkers to confidentially diagnose the cancer cells in early stages (5).
Discovering miRNAs has considerably improved the diagnostic and treatment complications (6). miRNAs are specific for any tissue. The miRNAs have been introduced as proper tumor markers for many reasons. Rosenfeld et al. indicated that the specificity of miRNAs is 90% (8). The expression of miRNAs in tumorous tissues is very different in comparison with healthy tissues. The miRNAs are involved in the carcinogenesis and their expression varies in different stages of cancer (6). Moreover, these biomarkers have good stability in freshly prepared tumorous tissues, paraffin-embedded tissue and peripheral blood (9). The miRNAs are small, endogenous, single-stranded, non-coding RNAs which include 17–25 nucleotides in length (10). MicroRNAs are involved in the regulation of gene expression in post-transcription level by specific interactions with their target mRNA. They regulate the gene expression by inhibiting the translation or cleaving the target mRNA through binding to 3' untranslated region (3' UTR) of a subset of mRNAs. miRNAs are involved in regulating the different cellular pathway such as apoptosis, angiogenesis, proliferation and differentiation. In-silico analyses indicated that miRNAs can regulate the expression of almost 30% of human proteins (11). When a malignancy occurs in a body tissue, the expression of miRNAs, in different tumors and therefore different stages of the disease will be increased or reduced (12).
When mutation occurs, increased expression of E2F1, a transcription factor, may increase transcription of miR-20a, which is a member of cluster 17-92. E2F1 inhibits genes involving in apoptosis and cell growth and its increased expression may led to cancer (13). However, there is less information about this mechanism. On the other hand, the role of miR-22 is not clear in carcinogenesis. A few studies have detected miR-22 as a tumor suppressor (14,15) and its expression has been increased in many cancers, such as bronchial epithelial cells (16). In addition, these miRNAs are involved in several cancers of gastrointestinal tract (17,18).
Due to the importance of investigate each population miRNAs alterations this study tries to investigate the expression rate of both miR-20a, as confirmed oncogene in various malignancies and miR-22 in cancerous gastric tissues in comparison with healthy adjacent tissues in a small available study group.
Methods
Samples
This is a case-control study conducted on 64 samples (32 tumorous tissues and 32 healthy adjacent tissues of the same patients as control group) of patients in the age group of 31 to 83 years, who did not receive any treatment prior to the study. Samples were prepared based on ethical principles and obtained by receiving a written consent from the patients (previously taken by the staff of Imam Khumeini Hospital, Tehran).
RNA extraction
In order to conduct Real Time PCR processes, the extracting total RNA from tumorous and healthy tissues was required. For this purpose, all prepared tissues were crushed by homogenizer. For disrupting cells and dissolving cell components, Tryzol (Invitrogen, USA) was added according to manufacturer’s instruction. In the next stage, chloroform was added and the sample was centrifuged at 12,000 ×g for 15 minutes at 4 °C. The supernatant containing RNA was isolated and placed into a new tube and the same volume of isopropanol was added. The obtained mixture was incubated at room temperature for 10 minutes and centrifuged within the previous conditions. Once more, the supernatant was removed and 1ml ethanol 75% was added to the remaining RNA pellet, then centrifuged at 7,500 ×g for 5 minutes at 4 °C. In the next stage, the alcohol was discarded and RNA pellet was dried at 55 °C for 10 min. Finally, RNA pellet was resuspended in RNase-free water and stored at −80 °C.
Measurement of miRNA expression
Real Time PCR processes were done applying ParsGenome’s miRNA amplification Kit (ParsGenome, Iran) based on the guidelines of the manufacturer as below:
PolyA polymerase enzyme addition
One point five µg of RNA was added to 2 µL buffer 10×, 1 µL ATP (10 mm), 0.5 µL Poly A enzyme and DEPC-treated water, then incubated at 37 °C for 10 min.
First-strand cDNA synthesis
Six µL of obtained polyadenylated RNA was mixed in 2 µL buffer 5×, 0.5 µL RT enzyme, 0.5 µL miRNA cDNA synthesis specific primer (15 pmol) and incubated at 42 °C for 15 min. For inactivating RT enzyme, the mixture was stored at 85 °C for 15 min.
Real-time PCR amplification:
Ten µL SYBR Green master mix, 1 µL miR specific primers (10 pmol, designed by Pars Genome Company), and 1 µg of diluted cDNA were mixed together. The thermal cycling conditions included:
Five min at 95 °C, 5 seconds at 95 °C, 20 seconds at 62 °C, and 30 second at 72 °C. Thermal cycling proceeded with 35 cycles. No template control (NTC) was used for controlling the contamination (19).
Results
Statistical analysis
In order to determine the expression differences between miRNAs in the tumorous and healthy adjacent tissues the average of ∆Ct (CTmiRNA − CT5srRNA) were compared by paired sample t-test and independent sample t-test was used for statistical analyses of miRNAs expression differences in different ages, genders and stages. The “fold change” was calculated using 2−∆∆CT formula: ∆∆CT = (CTmiRNA −CT5srRNA)tumoral tissues − (CTmiRNA − CT5srRNA)healthy adjacent tissues (20).
The clinicopathological characteristics of GC patients are indicated in Table 1. The results showed that there was no significant differences in miRNAs expression rate between the two age groups (patients ≤50 years old and patients >50 years old). Moreover, no significant difference in miRNAs expression between two gender groups was observed, which indicates that age and gender have no influence on these miRNAs expression rates. Statistical analyses indicated that no significant differences existed between miRNAs expression rate and the stage of disease as well (Table 1).
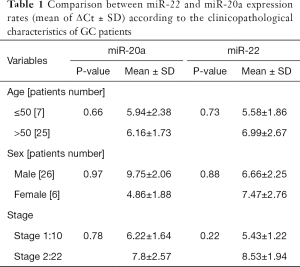
Full table
Expression of miR-22 in cancerous and healthy adjacent tissues
The value of Ct regarding miR-22 in tumorous tissues ranged from 14.08 to 22.31 and in healthy adjacent tissues from 14.6 to 20.1. Eighteen tumorous (56%) samples out of 32 samples indicated reduced expression in comparison with healthy adjacent tissues. The average of ∆∆CT for this miRNA was calculated 0.99 (Table 2). The calculated fold change of miR-22 expression was −1.9. This means that the expression rate of miR-22 was reduced 1.9-fold in the tumorous tissues. Statistical analyses indicated a significant difference in the expression rate of miR-22 in both study groups (P=0.014) (Table 3).
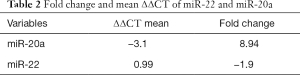
Full table

Full table
Expression of miR-20a in cancerous and healthy adjacent tissues
The results revealed that the value of Ct regarding miR-20a in tumorous tissues ranged from 12.69 to 20.27 and in healthy adjacent tissues from 16 to 26.5. Twenty-seven tumorous samples (84%) out of 32 samples indicated an increased expression in comparison with healthy adjacent tissues. The average of ∆∆CT for miR-20a was −3.16 (Table 2) and the expression fold change of miR-20a was 8.94. It means that the expression rate of miR-20a was increased 8.94-fold in the tumorous tissues (Table 2). Statistical analyses showed that a significant difference exists in the expression rate of miR-20a between both study groups (P<0.001) (Table 3).
Receiver-operator characteristic (ROC) curve analysis of expression levels of miR-20a and miR-22
ROC curve was obtained for both miRNAs. The area under the ROC curve (AUC) for each miRNA expresses its accuracy for differentiating GC and healthy tissues in terms of sensitivity and specificity. For miR-20a AUC (the area under the ROC curve) was equal to 0.879, indicating the high potency of this miRNA in differentiating GC and healthy tissues. However, for miR-22, the result was not satisfactory and therefore the data are not shown in this paper.
Discussion
Although there are many biomarkers like carcinoembryonic antigen (CEA), cancer antigen (CA) 9–19, and CA72-4, but they don’t have enough specificity and sensitivity (6). Therefore, biomarker investigations have been focused on miRNAs. Numerous miRNAs have been identified in GC development, but their underlying molecular mechanism in GC development is still poorly understood (21,22). Bioinformatics studies have indicated that more than 600 target genes for miR-20a and miR-19 have been known, including PTEN and RB2, and still more studies are required (13). As miRNA expression varies in different geographical regions, the current study investigates the role of these miRNAs in Iranian population.
Previous studies indicated an increased expression of miR-20a and this study confirms the results of the previous studies. The study conducted by Li et al. in 2013 on GC cell lines have shown that miR-20a, by inhibiting early growth response 2 (EGR2), causes proliferation and invasiveness of GC cells and the inhibitory effect of miR-20a can be reduced by up-regulating of EGR2 expression. Li indicated that there is an association between cancer metastasis and miR-20a expression while there is no relation with other clinicopathological parameters (23).
In the current study no significant differences were found between miR-20a expression and GC stage, age, and gender of the patients under study. This could be due to the limited sample size, the effect of the expression pattern of tumorous cells on healthy adjacent cells, and the selection of tumorous samples from patients at stages 1 and 2.
Moreover, fold change of this miRNA expression was reported 2.19 and 17.74 in GC (9,24) and 1.7 in colon cancer (12), while in present study fold change was calculated 8.94, indicating a possible relationship between miR-20a and GC. In addition, miR-20a expression showed a significant difference in both groups (P<0.001), which confirms the results of previous studies (22,24,25). In a study conducted by Liu et al. on serum samples, AUC for miR-20a was 0.89 and the sensitivity of this miRNA was indicated about 79% (26,27). In the present study, calculated AUC for miR-20a was 0.879 and the sensitivity of miR-20a was obtained about 84%, which indicates high potency of this miRNA for differentiating the healthy group from patient group.
miR-22 is also investigated in different cancers and its expression was reduced in several cancers such as ovarian cancer (28), colon cancer (29,30), and hepatocellular carcinoma (31) has been reported. Wang et al. in a study demonstrated the inhibitory role of miR-22 in GC (14). Moreover, downregulation of this miRNA is significantly correlated with poor prognosis in ovarian carcinoma patients (28). miR-22 inhibits the developing of liver cancer by targeting HDAC4 (histone deacetylase 4) (31). Because miR-22 is involved in the processes which is related to cancer such as cell growth, apoptosis, cell death and cell cycle, therefore this miRNA could be used for determining the prognosis of patients as well as for therapeutic purposes (14). Wang et al. in 2014 indicated that miR-22 prevents developing of tumor by targeting CD151 (15). In the present study miR-22 expression in the tumoral samples was significantly reduced in comparison with the healthy adjacent tissues (i.e., reduced expression in 18 cases out of 32 samples and increased expression in the remaining) which confirms the results of previous studies (28-31) and in the current study it was found that the expression fold change of this miRNA was −1.9 and the obtained sensitivity was 56%.
On the contrary, the expression of miR-22 has increased in epithelial bronchial cells and had a micro oncogenic role (16). Based on the previously mentioned, it is likely that miR-22 plays different roles in various cancers. Generally, the results obtained from miR-22 indicates that its expression has been reduced in tumorous tissues and confirms the results of previous studies (14,15,28). However, in contrast to previous studies, our results indicated no significant relationship between miR-22 expressions and GC stage. This could be due to the limited sample size, the effect of the expression pattern of tumorous cells on healthy adjacent cells and the selection of tumorous samples from patients at stages 1 and 2. Although previous studies have considered miR-22 as a good marker for treatment, monitoring and prognosis, however it seems that this marker requires more investigations and the results must be cautiously interpreted. Our results indicated that the expression of miR-22 had no significant difference between stages 1 and 2. Therefore, miR-22 may not be a good marker for determining prognosis, hence it requires more studies. On other hand, the sensitivity of 56% is a case that complicates the interpretation of results. Although there was a significant difference between the expression of this miRNA in tumorous and healthy adjacent tissues, however, such size of sensitivity should not be ignored. Moreover, there is less information about the mechanism of action and the effect of miR-20a in development of GC. However, the obtained results indicate that this miRNA is more likely to influence the development of GC and by more confidence, miR-20a could be used as a proper diagnostic marker.
Acknowledgements
Funding: This work was financially supported by Research and Technology Deputy of Hamadan University of Medical Sciences (Hamadan, Iran) with a grant number of 9408124320 and was a part of MSc thesis of ZJS.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was prepared based on ethical principles (ethics approval ID number: D/P/16/35/9/314) and obtained by receiving a written consent from the patient (previously taken by the staff of Imam Khumeini Hospital).
References
- Etemadi A, Sadjadi A, Semnani S, et al. Cancer registry in Iran: a brief overview. Arch Iran Med 2008;11:577-80. [PubMed]
- Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran: epidemiology and risk factors. Arch Iran Med 2009;12:576-83. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [Crossref] [PubMed]
- Dhalla F, da Silva SP, Lucas M, et al. Review of gastric cancer risk factors in patients with common variable immunodeficiency disorders, resulting in a proposal for a surveillance programme. Clin Exp Immunol 2011;165:1-7. [Crossref] [PubMed]
- Cooke CL, Torres J, Solnick JV. Biomarkers of Helicobacter pylori-associated gastric cancer. Gut Microbes 2013;4:532-40. [Crossref] [PubMed]
- Gao M, Yin H, Fei ZW. Clinical application of microRNA in gastric cancer in Eastern Asian area. World J Gastroenterol 2013;19:2019-27. [Crossref] [PubMed]
- Yang S, Chung HC. Novel biomarker candidates for gastric cancer. Oncol Rep 2008;19:675-80. [PubMed]
- Rosenfeld N, Aharonov R, Meiri E, et al. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol 2008;26:462-9. [Crossref] [PubMed]
- Yin Y, Li J, Chen S, et al. MicroRNAs as diagnostic biomarkers in gastric cancer. Int J Mol Sci 2012;13:12544-55. [Crossref] [PubMed]
- Allegra A, Alonci A, Campo S, et al. Circulating microRNAs: new biomarkers in diagnosis, prognosis and treatment of cancer Int J Oncol 2012;41:1897-912. (review). [PubMed]
- Aigner A. MicroRNAs (miRNAs) in cancer invasion and metastasis: therapeutic approaches based on metastasis-related miRNAs. J Mol Med (Berl) 2011;89:445-57. [Crossref] [PubMed]
- Wiemer EA. The role of microRNAs in cancer: no small matter. Eur J Cancer 2007;43:1529-44. [Crossref] [PubMed]
- Zhang B, Pan X, Cobb GP, et al. microRNAs as oncogenes and tumor suppressors. Dev Biol 2007;302:1-12. [Crossref] [PubMed]
- Wang W, Li F, Zhang Y, et al. Reduced expression of miR-22 in gastric cancer is related to clinicopathologic characteristics or patient prognosis. Diagn Pathol 2013;8:102. [Crossref] [PubMed]
- Wang X, Yu H, Lu X, et al. MiR-22 suppresses the proliferation and invasion of gastric cancer cells by inhibiting CD151. Biochem Biophys Res Commun 2014;445:175-9. [Crossref] [PubMed]
- Liu L, Jiang Y, Zhang H, et al. miR-22 functions as a micro-oncogene in transformed human bronchial epithelial cells induced by anti-benzo[a]pyrene-7,8-diol-9,10-epoxide. Toxicol In Vitro 2010;24:1168-75. [Crossref] [PubMed]
- Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ 2013;20:1603-14. [Crossref] [PubMed]
- Wang W, Li F, Zhang Y, et al. Reduced expression of miR-22 in gastric cancer is related to clinicopathologic characteristics or patient prognosis. Diagn Pathol 2013;8:102. [Crossref] [PubMed]
- Benes V, Castoldi M. Expression profiling of microRNA using real-time quantitative PCR, how to use it and what is available. Methods 2010;50:244-9. [Crossref] [PubMed]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008;3:1101-8. [Crossref] [PubMed]
- Xiao B, Guo J, Miao Y, et al. Detection of miR-106a in gastric carcinoma and its clinical significance. Clin Chim Acta 2009;400:97-102. [Crossref] [PubMed]
- Gigek CO, Chen ES, Calcagno DQ, et al. Epigenetic mechanisms in gastric cancer. Epigenomics 2012;4:279-94. [Crossref] [PubMed]
- Li X, Zhang Z, Yu M, et al. Involvement of miR-20a in promoting gastric cancer progression by targeting early growth response 2 (EGR2). Int J Mol Sci 2013;14:16226-39. [Crossref] [PubMed]
- Li X, Luo F, Li Q, et al. Identification of new aberrantly expressed miRNAs in intestinal-type gastric cancer and its clinical significance. Oncol Rep 2011;26:1431-9. [PubMed]
- Wang M, Gu H, Wang S, et al. Circulating miR-17-5p and miR-20a: molecular markers for gastric cancer. Mol Med Rep 2012;5:1514-20. [PubMed]
- Guo J, Miao Y, Xiao B, et al. Differential expression of microRNA species in human gastric cancer versus non-tumorous tissues. J Gastroenterol Hepatol 2009;24:652-7. [Crossref] [PubMed]
- Liu R, Zhang C, Hu Z, et al. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer 2011;47:784-91. [Crossref] [PubMed]
- Wan WN, Zhang YQ, Wang XM, et al. Down-regulated miR-22 as predictive biomarkers for prognosis of epithelial ovarian cancer. Diagn Pathol 2014;9:178. [Crossref] [PubMed]
- Yamakuchi M, Yagi S, Ito T, et al. MicroRNA-22 regulates hypoxia signaling in colon cancer cells. PLoS One 2011;6:e20291. [Crossref] [PubMed]
- Li J, Zhang Y, Zhao J, et al. Overexpression of miR-22 reverses paclitaxel-induced chemoresistance through activation of PTEN signaling in p53-mutated colon cancer cells. Mol Cell Biochem 2011;357:31-8. [Crossref] [PubMed]
- Zhang J, Yang Y, Yang T, et al. microRNA-22, downregulated in hepatocellular carcinoma and correlated with prognosis, suppresses cell proliferation and tumourigenicity. Br J Cancer 2010;103:1215-20. [Crossref] [PubMed]


