Gallbladder cancer: South American experience
Introduction
Gallbladder cancer (GBC) is a common disease in South America (1). It is possible that risk factors, molecular abnormalities and clinical behavior are different in comparison to GBC diagnosed in other regions. We present an overview of the situation of this disease in the area where we reside. Clinical characteristics, risk factors, genetic abnormalities and treatment approaches are described in order to picture the magnitude of this problem.
Epidemiology
Biliary tract cancers (BTC) include GBC, intrahepatic-cholangiocarcinoma (CC), extrahepatic-cholangiocarcinoma (EHCC) and some forms of ampullary cancer. Among them, GBC is the most prevalent cancer type in South America. Its incidence and mortality rates vary among areas; even within the same country remarkable differences in terms of mortality rates have been observed (1,2). Cancer registries are not well developed in some countries of South America, and in these cases mortality rates constitute an alternative to indirectly obtain information about the magnitude of this health problem. Furthermore, utilizing this approach is reliable because GBC is a highly lethal disease; therefore mortality rates can equalize incidence. This notwithstanding, caution is warranted because mortality rates due to GBC may be underestimated as well. In fact, in many South American countries the death certificates, which are the source for obtaining mortality rates, do not show that cancer (any type) was the cause of death. An example of such situation is reflected by the data on age-standardized mortality rates (ASMR) from Argentina vs. USA. In the former the ASMR due to cancer in general was 88 and 131/105/year for women and men, respectively (2). While in the later the ASMR were 145 and 208/105/year for women and men, respectively (3). This big difference in terms of ASMR can be explained, at least in part, by sub-registration of death from cancer.
Hepatobiliary tumors fall into two categories of the International Agency for Research on Cancer (IARC) report. Intrahepatic-CC and liver cancer are included in the ICD-10 C22 category, whereas GBC and EHCC in the ICD-10 C23-24. Intriguingly, ampullary cancer still remains unclassified in this report. In South America, the ASMR for tumors included within the category ICD-10 C22 are similar or below to that reported for other parts of the world (1). On the contrary, the geographic distribution of ASMR data for tumors belonging to the ICD-10 C23-24 category, in which GBC accounts for the majority of deaths, is substantially different. Furthermore, important variations in terms of ASMR in women have been found among South American countries (Table 1).

Full table
In Chile, and according to official statistics (4), a total of 23,716 deaths caused by GBC and EHCC have been reported for the period 2000–2012; among them 72.3% were women. Median ASMR was 15.04/105 women/year [interquartile range (IQR) 4.56)]. The highest ASMR were registered in the southern regions of Los Ríos (24.26; IQR 5.64), Los Lagos (23.69; IQR 4.14) and Aysén (18.29; IQR 7.78); while the lowest ASMR were described in the metropolitan region of Santiago de Chile (11.40; IQR 3.52) at the center, and Tarapacá at the north of the country (11.42; IQR 8.37). GBC and EHCC were the second leading cause of death from cancer in women in the whole country. On the other hand, the median ASMR for Chilean men was 5.52 (IQR 1.73), and the region of Aysen showed the highest ASMR (8.83; IQR 6.91).
In Brazil (5), GBC and EHCC accounted for 16,808 deaths in the period 2007–2012, and the majority corresponded to women (66.8%). The median national ASMR was 1.79/105 women/year (IQR 0.19). The highest ASMR were registered in the southern region of Rio Grande do Sul (2.08; IQR 0.16). Despite of this GBC and EHCC do not fall within the ten most common type of cancer causing deaths in every regions of the country, in both genders.
In Uruguay (6), at the southeast of South America, there were 1,058 deaths due to GBC and EHCC during the period 2006–2010, and 63.1% of them were women. The national ASMR for that period was 4.1/105 women/year.
Peru and Ecuador located at the central-western area of SA are two countries in which incidence rates are available. Age-standardized incidence rates (ASIR) were 7.5/105 women/year in Trujillo (Peru) and 7.4 in Quito (Ecuador) (7).
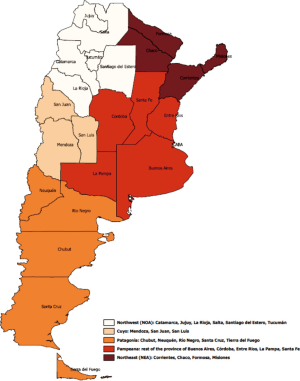
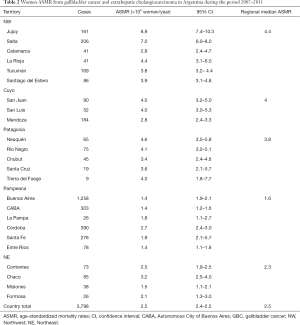
In Argentina, GBC and EHCC accounted for 5,262 (1.5%) of all cancer deaths in the period 2007–2011 (2). Out of them 3,806 (72.3%) occurred in women. The country overall ASMR was 2.5/105 women/year [95% confidence interval (CI), 2.4–2.5)] and 2.1/105 men/year (95% CI, 2.0–2.1). GBC and EHCC are among the ten most frequent (8th) cause of death from cancer in women, but not for men. The territory of Argentina can be divided in five geographic regions (Figure 1) which exhibit particular ethnical, political, historical, socio-economical characteristics, as well as differences in term of access to health care (8). Interestingly, the ASMR because GBC and EHCC varies between these regions (Table 2, Figure 2). The highest ASMR have been determined for the Northwest (NW) and the Patagonia regions, while the lowest values were estimated for the Northeast (NE) and Pampeana regions on the east side of the country.
Full table
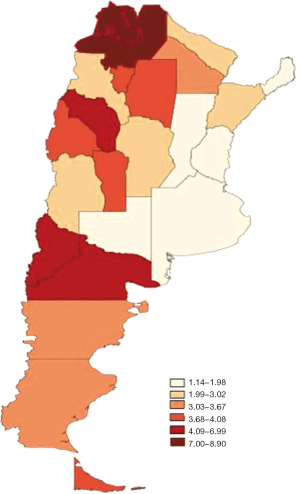
In the NW region, Jujuy showed an ASMR for women of 8.9 (95% CI, 7.4–10.3). Similarly, in the province of Salta the ASMR for women was 7.0 (95% CI, 6.0–8.0), occupying the third place among the leading causes of death from cancer in women in both provinces. In the province of La Rioja ASMR for women was 4.4 (95% CI, 3.1–6.0) being the fifth leading cause of cancer death in women.
In the province of Neuquén (Patagonia region) the ASMR for GBC and EHCC was 4.6 (95% CI, 3.5–5.8), placing these tumor types at the 8th leading cause of cancer death in women. In Rio Negro, belonging to the same region, women ASMR was 4.1 (95% CI, 3.2–5.1), reaching the 7th place in the leading causes of death from cancer in women.
The lowest ASMRs for GBC and EHCC in women (1.4) were registered in the provinces of Entre Rios and Buenos Aires, and the Autonomous City of Buenos Aires itself (CABA), all of which belong to the eastern Pampeana region.
In summary, the data about mortality rates due to GBC indicate that the incidence of this tumor type in South America is distinct in different geographic areas. While in the eastern countries, like Uruguay and Brazil and the NE region of Argentina the data on ASMR due to GBC are similar to those of the United States of America and Western Europe (9), in Chile and western areas of Argentina the same measurements yielded higher values. In western countries such as Peru and Ecuador ASIR (which equalizes ASMR) were high. These data suggest that there is a widely extended territory near the Andes Mountains in which people are at high risk of developing GBC.
Etiology and risk factors for GBC in South America
The pathogenesis of GBC is not completely elucidated; however it is known that genetics and environmental factors play important roles in the initiation, promotion and progression of the disease (Table 3).

Full table
Non-manageable risk factors
GBC mortality increases with aging. In Argentina, in the period 2007–2011 the number of deaths in women was 70, 340, 456 and 547 in the 40–44, 55–59, 64–69 and 75–79 year groups respectively; in men deaths due to GBC were 31, 232, 367 and 415 in the same age groups, respectively (2). In the USA, in the period 2008–2012, a similar distribution of deaths due to GBC has been observed in both sexes (3). We could speculate that this epidemiological characteristic responds to the accumulation of hits which are required by the process of carcinogenesis.
As mentioned before this tumor type is more prevalent among women, and it has been proposed a link between this feature and the estrogen levels. Females having early menarche, late menopause, multiple pregnancies and childbirths appear to have an increased risk of developing GBC according to a case-control study from India (19). Interestingly, estrogens increase the formation of gallstones, mainly by elevating biliary cholesterol (20).This could explain, at least partially, why GBC is more prevalent in women.
The contribution of the genetic background as predisposing factor for GBC emerges from the analysis of migratory phenomenon from high incidence areas in Chile and Bolivia to Argentina. The NW region in Argentina shares borders with the Chilean region of Antofagasta, and GBC mortality rates are similar. The original natives belonged to the Omaguacas, Atacamas and Diaguitas tribes, who have particular cultural, social, dietary habits and obviously specific genomic characteristics. Some of them changed dramatically after the Spanish conquest, but resilience processes in valleys, however, could preserve them to certain extent. Furthermore, this ethnic reality has been maintained until today by permanent migrations from Bolivia to Argentina, to the point that today about 350,000 Bolivians and their natural descendants (Argentinean) live in Argentina, mostly in Jujuy, Salta and Buenos Aires (21). Similar situation is observed in Patagonia, another high incidence region in Argentina, where the ancestral Pewenches were invaded by Mapuches, coming from the south of Chile, establishing a bidirectional population flow that persists nowadays (22). Therefore, and given the historic and demographic evidences connecting populations at both sides of the Andes, it is tempting to speculate that genetic background common to those persons predispose them to GBC development. Of course, thorough investigations are needed to verify this hypothesis, but a recent study on the genetic variants of the Arsenic+++ Methyl Transferase gene (AS3MT) provided the first evidence of human adaptation to a toxic chemical (23). It is well known that chronic arsenic poisoning may lead to skin cancer, and this study revealed that there are, among the population residing in the Andean area of Province of Salta, women carrying certain haplotype of the AS3MT gene, involved in arsenic metabolism, that protect them from the toxic effects of arsenic. Additional evidence for the notion that genetic factors may contribute to GBC development comes from a case-control study in a Chilean population about lipid metabolism-related genes. In fact, the rs693 polymorphism of the Apolipoprotein B gene and the rs708272 polymorphism of the Cholesteryl-Ester-Transfer-Protein gene were associated with an increased risk of GBC (OR 5.04; 95% CI, 1.43–17.8) (10).
Manageable risk factors
Cholelithiasis which leads to chronic cholecystitis remains as the most important risk factor for the disease worldwide. Gallstones are present in 45–100% of the cases (24), and mucosal changes from cholecystitis, hyperplasia, metaplasia, dysplasia, to carcinoma correlate positively with the weight, and size of the stones (25). The association between gallstone disease and GBC is even stronger if this has a longstanding history as shown by Serra et al. in a case-control study of 228 persons from Chile. They reported that 15% of cancer cases had a history of gallstone disease longer than 24 years compared to only 4% among control cases (OR 11; 95% CI, 1.4–85.2) (14).
In the case-control study mentioned above Serra et al. also found that red chili pepper consumption was associated with GBC (together with very low socioeconomic status and longstanding gallstone disease). The association resisted the statistic challenge by multivariate analysis with ORs of 3.2 (95% CI, 1.7–5.9). Since this is a retrospective analysis it is subjected to biases, including recall bias, a common limitation when using food frequency questionnaires. In support of that finding other studies reported an association of chili pepper consumption with stomach, liver and esophageal cancer (26-28). Furthermore, in the search for the potential etiological factor related to this dietary habit, several investigations have shown elevated concentration of aflatoxin in red chili peppers consumed by populations having high GBC incidence in the Andean areas of Chile, Bolivia and Peru (15,16). Nogueira et al. (17) also analyzed this association by performing a case-control study including 112 Chilean men and women (not distinguishing gender), and they found that patients with GBC had higher levels of aflatoxin B1-DNA adducts in peripheral blood than healthy control individuals (OR 13.2; 95% CI, 4.3–47.9) and control individuals with gallstones (OR 9.4; 95% CI, 2.8–37.2). In summary, there is evidence to consider red chili pepper, a frequently consumed food in some regions of South America, as a risk factor for GBC. This, in turn, could be mediated by the mutagen aflatoxin, a contaminant of red chili pepper. However, due to the limitations of the studies outlined before, additional research is required to confirm the association of red chili pepper consumption and GBC development.
Very low socioeconomic status was found associated to GBC in the study by Serra et al. described above (14), with a ORs of 6.3 (95% CI, 1.7–23.0) after multivariable analysis. It is conceivable that people with very low socioeconomic status have less access to the health care system and, therefore is more likely to have longstanding gallstone disease.
A study performed in Mexico and Bolivia showed that patients diagnosed for typhoid disease had a twelvefold higher risk of developing GBC (11) which is in agreement with studies carried out in USA and Asia (12,18).
Other possible risk factors are chronic biliary tract infection, diet, elevated body mass index, smoking or chewing tobacco and genetic factors (11,13,19,29).
In summary, age, gender (female) and the genetic make-up appear as important non modifiable risk factors for GBC, whereas cholelithiasis, typhoid disease, as well as consumption of red chili pepper contaminated with aflatoxin and very low socioeconomic status emerge as risk factors on which we may be able to intervene.
Molecular pathology of GBC
Castillo et al. (30) proposed that the malignant transformation of the gallbladder may occur through two alternative pathways (models): the sequential change from dysplasia to carcinoma and the adenoma-carcinoma model. The first postulates that the gallbladder epithelium undergoes metaplasia as an adaptive response to chronic irritation and inflammation. In fact, such change is found in approximately 50% of the patients with cholecystitis, and, eventually, on top of the metaplasia, the dysplasia may develop, which progress to carcinoma. The second model postulates that GBC originates through the malignant transformation of glandular tumors, such as benign adenomas.
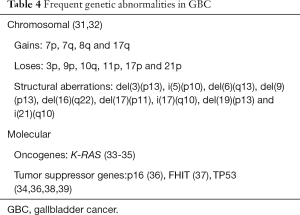
Regardless the course of the disease, it is accepted that, like in the vast majority of neoplastic processes, genetic changes play a pivotal role in the development of GBC (31). The most frequent genetic abnormalities in GBC are depicted in Table 4. Classical cytogenetic studies performed on tumor samples of patients from low endemic areas, more than a decade ago, showed that the chromosome profile of GBC is complex. The tumors contain multiple clones, and the chromosome profile was characterized by gains of the chromosome arms 7p, 7q, 8q and 17q, and loses of 3p, 9p, 10q, 11p, 17p and 21p. Furthermore, several recurrent chromosome aberrations were detected, including del(3)(p13), i(5)(p10), del(6)(q13), del(9)(p13), del(16)(q22), del(17)(p11), i(17)(q10), del(19)(p13) and i(21)(q10), suggesting that these regions contain genes involved in the development of GBC (32).
Full table
Roa et al. (40), performed studies aimed to detect genomic imbalances in GBC from Chile, and to find whether there is any association with the prognosis of the patients. Flow cytometry analysis showed that 24% of the tumors (29/120) were aneuploidy. However five years survival was not significantly worse among these patients compared to those with diploid tumors. Molecular studies has allowed to identify, among others, tumor suppressor genes, oncogenes, genes involved in DNA repair which play important roles in the pathogenesis of GBC. Furthermore, and based on the analysis of genetic abnormalities of different lesions in patients from Chile, it has been possible to propose a sequence of molecular events implicated in GBC development (30). Activation of the members of the RAS family of genes (H-RAS, N-RAS y K-RAS) is perhaps one of the most frequent events in human neoplasia. However the data on activating mutation of K-RAS in this tumor type are quite variable. For example, Pai et al. (33) reported that only two out 29 (7%) analyzed tumors of patients from USA exhibited K-RAS codon 12 mutations, while the frequency of mutation in tissue samples of patients from India and Korea was near to 50% and 20%, respectively (36,41). Interestingly, differences in the frequency of K-RAS mutation were also detected between patients from the NW of Argentina and from Bolivia; despite both populations subjected to genetic analysis belonged to the same geographic area. The study performed to identify the status of K-RAS in GBC patients from Bolivia showed that one out of 36 (2.8%) had activating mutations of the gene (34), while patients from the NW of Argentina exhibited higher frequency of this gene abnormalities. In fact, in a study including 58 patients of BTC, with a majority of GBC, 23 patients (39%) had amplification and 8 (17%) had mutation of the K-RAS gene (unpublished data). Even more, in 21 invasive GBC samples from Chile RAS mutations were found in only 2 (10%) (35). This variation among high risk areas suggests that environmental factors and the genetic background of the people may be related to such differences.
Tumor suppressor genes FHIT mapping on chromosome 3p14, p16 on 9p21 and TP53 on 17p13 are among the most frequently found altered in GBC, and this is consistent with the data about imbalances of their genomic regions. Kim et al. (36) have detected deletions of the chromosome region 9p21 and methylation of the promoter region of the p16 gene in 48% of GBC. This correlated with loss of the protein expression in 50% of tumors, determined by immuno-histochemistry. Methylation in the promoter region of the FHIT and p16 genes has been also examined by Roa et al. (37) in GBC samples of patients from Chile. Methyl-specific PCR showed that 30% of the tumors had abnormal methylation of the promoter region of the FHIT gene and 20% of p16 gene promoter, and this was in agreement with an altered immune-histochemical pattern of their protein product. Considering the number of scientific papers available in the literature, TP53 is the tumor suppressor gene most thoroughly investigated in this tumor type (38). The authors mentioned above demonstrated that the percentages of tumors with deletions of the locus (81%) and mutations of exons 6–8 (67%) are in agreement with the high incidence (66%) of overexpression of the mutated protein in tumor cells (36). The TP53 gene status has been also the focus of genetic studies performed on samples from patients from Chile and Bolivia. Unlike to what happens to the K-RAS gene, the status of abnormalities of this gene is similar among high risk GBC regions. Mutations of TP53 were found in more than 50% of tumor samples from Bolivia and Chile. However, differences in the type of mutations were found, and despite of the fact that exons 5 and 8 were affected, only a few mutations occurred in hot-spot codons (34,39). In another study from Chile, Wistuba et al. (35), found that deletion of the TP53 locus was an early event present in 58%, 85% and 91% of dysplastic tissue, carcinoma in situ and invasive carcinoma, respectively.
We have analyzed the expression of the KIT protein in GBC samples from our area (42). Contrary to the data obtained from a series of patients from USA (43), which showed expression of the protein in the majority of tumors, only three out of the 50 tumor samples we subjected to standard protein immuno-detection, exhibited positive reaction. Interestingly all three positive were poorly differentiated or undifferentiated tumors, raising the hypothesis that this metabolic pathway could be utilized by a subset of aggressive GBC.
Roa et al. (44) analyzed the presence of microsatellite instability (MSI) in 59 samples from patients with GBC and 22 with chronic cholecystitis from Temuco, a high risk area for GBC in Chile, and 6 out of the 59 (10%) samples showed high MSI. Interestingly none of chronic cholecystitis cases exhibited MSI, whereas it was present in 83% (5/6) of surrounding dysplasia, in 33% (2/6) of surrounding intestinal metaplasia, and with equal proportion in early and advanced stages GBC samples, suggesting the hypothesis that mismatch repair deficiency is an early event in gallbladder carcinogenesis. In contrast MSI has been rarely found in samples from European GBC patients (45). Analysis of MSI as a sign of mismatch repair deficiency is even more interesting because this molecular abnormality has recently been found to be a predictor of impressive responses to anti PD-1 therapy, including patients with BTC (46).
The Gastrointestinal Oncology Latin-American Intergroup (ILOGI) analyzed the transcriptional level of different genes as predictive or prognostic factors in 54 BTC samples (including GBC, CC and ampullary cancer). Among others the mRNA level of FBXW7 a gene that encodes an ubiquitin ligase that interacts with oncoproteins, correlated with progression free survival (PFS) and overall survival (OS) in 19 patients (47). Median PFS was 4.9, 7.6 and 26.9 months for tumors in the lowest, middle and upper tercile of expression level. The corresponding median OS were 6.2, 8.8 and not reached. Mutational analysis of the FBXW7 gene on 30 samples did not reveal structural anomalies (unpublished data). This is in contrast with a finding by Akhoondi et al. (48) who reported inactivating mutations in 7/20 (35%) non-South-American CCs. It would be interesting to expand these analyses to a higher number of cases in order to confirm FBXW7 mRNA level as a prognostic factor in BTC, and to confirm if FBXW7 inactivating mutations have different prevalence in CCs from South America respect to tumors from other regions.
Given the much higher incidence of GBC in the Western area of South America, our results in tumors from this area reinforce the notion that particular environmental or genetic factors are leading to specific genetic alterations which may result in different molecular pathways.
Clinical experience and clinical trials in South America
In South America, as expected, the management of the patients with GBC has been also carried out by surgery, chemotherapy (CHT), radiotherapy or the combination of them. Consequently a number of studies, including clinical series as well as clinical trials have been performed aimed to gain insights into the different treatment options for GBC in South America (Tables 5,6).

Full table
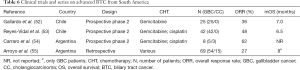
Full table
Before describing the studies on early stage GBC one should keep in mind that the majority of early stage GBC cases are diagnosed as an incidental finding after a simple laparoscopic cholecystectomy. Under this scenario, it is recommended performing an extended cholecystectomy. This surgical procedure starts with an initial examination of the peritoneal cavity and the retroperitoneal lymph nodes. If no tumor is found it continues with a regional lymphadenectomy, a wedge resection of the gallbladder bed (usually including segments IVb and V of the liver), and resection of port sites. Supporting evidence for such aggressive approach comes from the facts that in tumors staged T2 (invasion without penetration of serosa) lymph node spread occurs in 48% of patients (56,57) and for stage T3 lesions (perforation of serosa or direct invasion to liver and/or one adjacent organ) the spread to lymph nodes occurs in 72% of patients (57).
Manterola et al. (49) treated 40 patients from Chile with extended cholecystectomy followed by CHT with 5-fluorouracil/leucovorin. According to the TNM Classification of Malignant Tumors (58), the depth of penetration of their tumors wereT1/T2/T3/T4 in 8/12/12/8 patients, respectively, and the 5 year OS was 50%. Similarly, Lendoire et al. (50) reported 53% 5 year OS among 24 GBC patients from Argentina, including 1 T1, 12 T2 and 11 T3, who were treated with extended cholecystectomy and CHT. A large retrospective study was recently presented by González et al. at the 2015 Latin-American Symposium of Oncologic Gastroenterology (SLAGO) in Viña del Mar (Chile) (51). These authors analyzed the outcome of 95 patients with GBC, including mostly very early stage tumors (27 T1, 34 T2 and 34 T3). Among them, only 49 patients (52%) were subjected to extended cholecystectomy and the remaining received simple cholecystectomy. Adjuvant CHT and 3D radiation therapy were utilized. Of the total, 42% of the patients were alive after 5 years. However, clear differences in term of OS were achieved by patients who received either extended or simple cholecystectomy, 55% of those who underwent the extended procedure were alive after 5 years, whereas only 29% of those who received simple cholecystectomy were alive after 5 years. Moreover, in this study the performance of the patients who underwent extended cholecystectomy was also evaluated in relationship with the number of lymph nodes examined. Five year OS was 67% for those patients who had four or more lymph nodes examined, whereas it descended to 47% among patients with less than four lymph nodes subjected to histopathology exam. Although the data were obtained retrospectively, they show the importance of extended cholecystectomy as the best treatment alternative for those cases with early stage GBC. Another important point that can be deduced from the analysis of the surgical series presented here is that adjuvant CHT after a complete resection of GBC is indicated in the majority of cases in South America. This is in agreement with the literature reporting that adjuvant CHT is utilized by 70% of the centers worldwide for the treatment of this type of cancer (59). However, since this decision is based on low level evidence it must be confirmed by the ongoing phase 3 trials (in Japan, France and the United Kingdom) which are testing the hypothesis that surgery and adjuvant CHT cure more patients than surgery alone.
Finally, it is important to mention that reports from the United States of America and other countries from Asia and Europe showed that the 5 year OS rates varied between 54–100% for T2, 16–63% for T3, and 8–25% for T4 disease (60), which are to certain extent comparable with the OS data from patients with early stage GBCs from South America. Therefore, we can assume that the treatment strategies established in our patients are well supported by the clinical evidence.
As regard to advanced GBC, several attempts to treat the patients with CHT have been performed. Gallardo et al. (52) carried out a phase 2 trial with first line gemcitabine in 26 patients with advanced GBC from Chile, and reported that an overall response rate (ORR, the sum of partial and complete responses) was obtained in 9/25 (36%) and that stable disease was achieved by 6/25 (25%) patients, while the median OS was 7 months. More than a decade ago, Reyes-Vidal et al. (53) presented at the 2004 ASCO Gastrointestinal (GI) Meeting results of a phase 2 trial with gemcitabine combined with cisplatin in the first line setting in 42 patients with advanced GBC from Chile. ORR was achieved by 20 out of the 42 (48%) patients, and median survival was 6.5 months. Similar treatment protocol was administrated to a group of 8 patients with locally advanced and metastatic GBC and CC from Argentina, and response was obtained in 5 of them (62%); however data about the OS was not reported (54). We have surveyed the clinical data from 173 patients with GBC and CC from the NW region of Argentina, attended by physicians’ members of the ILOGI group. There were clear gender differences between the two clinical entities, GBC was more prevalent among women and CC was evenly distributed. Furthermore, GBCs were almost always detected at earlier stages than CC, which could be due to the fact that GBC is diagnosed as an incidental finding. More important, 69 patients (54 GBC and 15 CC) were assessed for their response to gemcitabine or cisplatin-based CHT, and overall response was obtained in 16 out of 54 (30%) GBC patients and 3 out 15 (20%) CC patients. The median OS for the GBC patients was 8 months (55).
In these three trials and the retrospective series the ORR ranged from 27% to 62%. However, and despite the good response rates, the OS observed among these patients was dismal. Similarly, the OS in advanced GBC patients from other regions of the world is poor with no evidence of cure in any subgroup of patients (61,62). Therefore, new treatment strategies for advanced GBC are required. This could be accomplished by the identification of new targets and the appropriate therapy. In fact recent analyses in tissue samples from GBC in 100 patients found between 1 and 2 potentially targetable genomic alterations per tumor, among them ERBB2, PIK3CA, CDKN2A/B and KRAS abnormalities (63,64).
Since no single test for the early diagnosis has been shown to decrease mortality, it is reasonable to think that prophylactic simple cholecystectomy in a population at high risk for GBC in endemic areas could be beneficial, in particular for patients with gallstone disease who live in western South America. In fact, and in agreement with this notion, the Ministry of Health of Chile published a clinical guideline for the management of this type of patients, in which the use of prophylactic cholecystectomy in the population with gallstone disease, regardless of the presence of symptoms is highly recommended (65). In contrast, prophylactic cholecystectomy is not recommended in the majority of patients with asymptomatic gallstone disease except for select groups (66).
In summary, GBC is a frequent disease in many western regions of South America, with a dismal prognosis in the majority of patients. There is an urgent need of developing high quality cancer registries in these regions, to perform basic research and clinical trials, which can contribute to the better management of the patients.
Conclusions
GBC affects more frequently women in many western regions of South America. It represents a significant challenge to the public health system because this tumor type is among the leading causes of death due to cancer in these territories. It is likely that genetic, dietary habits and environmental factors contribute to the pathogenesis of this disease. Some differences in term of RAS, TP53, FHIT and p16 genes abnormalities have been found in GBC from South America compared to tumors from other regions. However, and similarly to other countries, extended cholecystectomy, and eventually adjuvant CHT, is the standard procedure for the treatment of resectable tumors, while the combination of gemcitabine and cisplatin is frequently administrated as the first line CHT for the treatment of advanced GBC in South America. In contrast to other regions, prophylactic cholecystectomy is a common practice in many western areas of South America. Due to the high incidence and the dismal outcome of the patients with GBC comprehensive and high quality cancer registries and more translational research should be carried out in order to minimize the negative impact that this disease has among South Americans.
Acknowledgements
Funding: Parada LA was funded by the National Council of Sciences and Technologies(CONICET); Argentine Agency for Science and Technology. Grant ANPCyT – FONCyT, PICT-2011-1897.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer 2013. Available online: http://globocan.iarc.fr, accessed on day/month/year
- Abriata GM, Roques LF, Macías G, et al. Cancer Mortality Atlas. Argentina. 2007-2011. Instituto Nacional del Cáncer. Ministerio de Salud de la Nación. Available online: http://www.msal.gov.ar/inc/images/stories/downloads/publicaciones/29-Atlas-de-mortalidadopt.pdf
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review 1975-2012, National Cancer Institute. Bethesda, MD. Available online: http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015.
- Principales causas de muerte según región, sexo y año de defunción. Departamento de Estadísticas e Información de Salud (DEIS) Chile, 2000-2012. Available online: http://www.deis.cl/?p=2541
- Atlas on Line 2014. Instituto Nacional del Cáncer, José Alencar Gomes Da Silva. Available online: https://mortalidade.inca.gov.br/
- Barrios E, Alonso R, Garau M, Musetti C. Situación epidemiológica del Uruguay en relación al cáncer. Registro Nacional de Cáncer, Comisión Honoraria de Lucha Contra el Cáncer. 2014. Available online: http://www.comisioncancer.org.uy/categoria_53_1.html
- Curado MP, Edwards B, Shin HR, et al. Cancer Incidence in Five Continents, Vol. IX. Lyon: IARC, 2007. Available online: http://www.iarc.fr/en/publications/pdfs-online/epi/sp160/
- Instituto Nacional de Estadísticas y Censos. Población. Available online: http://www.indec.gov.ar/nivel2_default.asp?seccion=P&id_tema=2
- Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelitiasis and cancer. Gut Liver 2012;6:172-87. [Crossref] [PubMed]
- Báez S, Tsuchiya Y, Calvo A, et al. Genetic variants involved in gallstone formation and capsaicin metabolism, and the risk of gallbladder cancer in Chilean women. World J Gastroenterol 2010;16:372-8. [Crossref] [PubMed]
- Strom BL, Soloway RD, Rios-Dalenz JL, et al. Risk factors for gallbladder cancer. An international collaborative case-control study. Cancer 1995;76:1747-56. [Crossref] [PubMed]
- Nagaraja V, Eslick GD. Systematic review with meta-analysis: the relationship between chronic Salmonella typhi carrier status and gall-bladder cancer. Aliment Pharmacol Ther 2014;39:745-50. [Crossref] [PubMed]
- Rai R, Sharma KL, Misra S, et al. PSCA gene variants (rs2294008 and rs2978974) confer increased susceptibility of gallbladder carcinoma in females. Gene 2013;530:172-7. [Crossref] [PubMed]
- Serra I, Yamamoto M, Calvo C, et al. Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a chilean population. Int J Cancer 2002;102:407-411. [Crossref] [PubMed]
- Tsuchiya Y, Terao M, Okano K, et al. Mutagenicity and mutagen of the red chili pepper as gallbladder cáncer risk factor in chilean women. Asian Pac J Cancer Prev 2011;12:471-6. [PubMed]
- Asai T, Tsuchiya Y, Okano K, et al. Aflatoxin contamination of red chili pepper from Bolivia and Peru, countries with high gallbladder cancer incidence rates. Asian Pac J Cancer Prev 2012;13:5167-70. [Crossref] [PubMed]
- Nogueira L, Foerster C, Groopman J, et al. Association of aflatoxin with gallbladder cancer in Chile. JAMA 2015;313:2075-7. [Crossref] [PubMed]
- Welton JC, Marr JS, Friedman SM. Association between hepatobiliary cancer and typhoid carrier state. Lancet 1979;1:791-4. [Crossref] [PubMed]
- Pandey M, Shukla VK. Lifestyle, parity, menstrual and reproductive factors and risk of gallbladder cancer. Eur J Cancer Prev 2003;12:269-72. [Crossref] [PubMed]
- Everson GT, McKinley C, Kern F Jr. Mechanisms of gallstone formation in women. Effects of exogenous estrogen (Premarin) and dietary cholesterol on hepatic lipid metabolism. J Clin Invest 1991;87:237-46. [Crossref] [PubMed]
- Censo de Población y Vivienda. Instituto nacional de estadísticas y censos (INDEC). Argentina 2010. Available online: http://www.censo2010.indec.gov.ar/novedades/novedades_detalle.asp?id=20983
- Government from Chile. Informe de la Comisión Verdad Histórica y Nuevo Trato con los Pueblos Indígenas. Presidential Commitee for Native People Affairs (ed). First edition, Santiago de Chile, 2008. Available online: http://www.corteidh.or.cr/tablas/27374.pdf
- Schlebusch CM, Gattepaille LM, Engström K, et al. Human Adaptation to arsenic-rich environments. Mol Biol Evol 2015;32:1544-55. [Crossref] [PubMed]
- Pandey M. Risk factors for gallbladder cancer: a reappraisal. Eur J Cancer Prev 2003;12:15-24. [Crossref] [PubMed]
- Mathur SK, Duhan A, Singh S, et al. Correlation of gallstone characteristics with mucosal changes in gall bladder. Trop Gastroenterol 2012;33:39-44. [Crossref] [PubMed]
- López-Carrillo L, Hernández Avila M, et al. Chili pepper consumption and gastric cáncer in Mexico: a case-control study. Am J Epidemiol 1994;139:263-71. [PubMed]
- Archer VE, Jones DW. Capsaicin pepper, cancer and ethnicity. Med Hypotheses 2002;59:450-7. [Crossref] [PubMed]
- Ghadirian P, Ekoé JM, Thouez JP. Food habits and esophageal cancer: an overview. Cancer Detect Prev 1992;16:163-8. [PubMed]
- Matsuba T, Qiu D, Kurosawa M, et al. JACC Study Group. Overview of epidemiology of bile duct and gallbladder cancer focusing on the JACC Study. J Epidemiol 2005;15 Suppl 2:S150-6. [Crossref] [PubMed]
- Castillo J, García P, Roa J. Alteraciones genéticas en lesiones preneoplásicas y neoplásicas de la vesícula biliar. Rev Med Chile 2010;138:595-604. [Crossref] [PubMed]
- Parada LA, Hallén M, Tranberg KG, et al. Frequent rearrangements of chromosomes 1, 7, and 8 in primary liver cancer. Genes Chromosomes Cancer 1998;23:26-35. [Crossref] [PubMed]
- Gorunova L, Parada LA, Limon J, et al. Nonrandom chromosomal aberrations and cytogenetic heterogeneity in gallbladder carcinomas. Genes Chromosomes Cancer 1999;26:312-21. [Crossref] [PubMed]
- Pai RK, Mojtahed K, Pai RK. Mutations in the RAS/RAF/MAP kinase pathway commonly occur in gallbladder adenomas but are uncommon in gallbladder adenocarcinomas. Appl Immunohistochem Mol Morphol 2011;19:133-40. [Crossref] [PubMed]
- Asai T, Loza E, Roig GV, et al. High frequency of TP53 but not K-ras gene mutations in Bolivian patients with gallbladder cancer. Asian Pac J Cancer Prev 2014;15:5449-54. [Crossref] [PubMed]
- Wistuba II, Sugio K, Hung J, et al. Allele-specific mutations involved in the pathogenesis of endemic gallbladder carcinoma in Chile. Cancer Res 1995;55:2511-5. [PubMed]
- Kim YT, Kim J, Jang YH, et al. Genetic alterations in gallbladder adenoma, dysplasia and carcinoma. Cancer Lett 2001;169:59-68. [Crossref] [PubMed]
- Roa JC, Anabalón L, Roa I, et al. Promoter methylation profile in gallbladder cancer. J Gastroenterol 2006;41:269-75. [Crossref] [PubMed]
- Chan E, Berlin J. Biliary tract cancers: understudied and poorly understood. J Clin Oncol 2015;33:1845-8. [Crossref] [PubMed]
- Yokoyama N, Hitomi J, Watanabe H, et al. Mutations of p53 in gallbladder carcinomas in high-incidence areas of Japan and Chile. Cancer Epidemiol Biomarkers Prev 1998;7:297-301. [PubMed]
- Roa I, de Aretxabala X, Fuentealba P, et al. DNA content and survival in subserous gallbladder carcinoma. Rev Med Chile 2004;132:794-800. [PubMed]
- Kazmi HR, Chandra A, Nigam J, et al. Prognostic significance of K-ras codon 12 mutation in patients with resected gallbladder cancer. Dig Surg 2013;30:233-9. [Crossref] [PubMed]
- Arroyo GF, Acosta G, Monteros Alvi M, et al. Prevalence of KIT expression in human tumors: gallbladder cancer. J Clin Oncol 2005;23:5268-71; author reply 5271-2. [Crossref] [PubMed]
- Aswad B, Constantinou M, Iannitti D. KIT is a potential therapeutic target for biliary carcinomas. Proc Am Soc Clin Oncol 2002;21:103.
- Roa JC, Roa L, Correa P, et al. Microsatellite instability in preneoplastic and neoplastic lesions of the gallbladder. J Gastroenterol 2005;40:79-86. [Crossref] [PubMed]
- Saetta AA. K-ras, p53 Mutations, and Microsatellite Instability (MSI) in Gallbladder Cancer. J Surg Oncol 2006;93:644-9. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch repair deficiency. The New Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Arroyo GF, Kaen D, Salvatierra A, et al. F-box and WD repeat domain-containing 7 (FBXW7) mRNA and outcome in biliary tract cancer. J Clin Oncol 2012;30:abstr e14521.
- Akhoondi S, Sun D, von der Lehr N, et al. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res 2007;67:9006-12. [Crossref] [PubMed]
- Manterola C, Vial M, Roa JC. Survival of a cohort of patients with intermediate and advanced gall bladder cancer treated with a prospective therapeutic protocol. Acta Cir Bras 2010;25:225-30. [Crossref] [PubMed]
- Lendoire JC, Gil L, Duek F, et al. Relevance of residual disease after liver resection for incidental gallbladder cancer. HPB (Oxford) 2012;14:548-53. [Crossref] [PubMed]
- González M, Silva S, Folch P, et al. Radio-quimioterapia adyuvante en pacientes con cáncer de vesícula biliar: experiencia Instituto Oncológico - Viña del Mar, Chile. Latinamerican Symposium of Oncologic Gastroenterology (SLAGO) 2015 Oral presentation.
- Gallardo JO, Rubio B, Fodor M, et al. A phase II study of gemcitabine in gallbladder carcinoma. Ann Oncol 2001;12:1403-06. [Crossref] [PubMed]
- Reyes-Vidal JM, Gallardo J, Yanez E, et al. Gemcitabine (Gem) and cisplatin (CIS) in the treatment of patients (pts) with unresectable or metastatic gallbladder cancer: results of the phase II GOCCHI study 2000-13. Proc Am Soc Clin Oncol 2003;22:abstr 1095.
- Carraro S, Servienti PJ, Bruno MF, et al. Gemcitabine and cisplatin in locally advanced or metastatic gallbladder and bile duct adenocarcinomas. Proc Am Soc Clin Oncol 2001;20:abstr 2333.
- Arroyo G, Carballido M, Kaen D, et al. Cholangiocarcinoma (C) and gallbladder cancer (G): two different diseases. Analysis of 173 patients. Ann Oncol 2010;21:P 0259.
- Bartlett DL, Fong Y, Fortner JG, et al. Long term results after resection for gallbladder cancer: implications for staging and management. Ann Surg 1996;224:639-46. [Crossref] [PubMed]
- Tsukada K, Hatakeyama K, Kurosaki I, et al. Outcome of radical surgery for carcinoma of the gallbladder according to the TNM stage. Surgery 1996;120:816-21. [Crossref] [PubMed]
- Sobin LH, Gospodarowicz MK, Wittekind C. editors. International Union Against Cancer (UICC). TNM Classification of Malignant Tumors. 7th ed. Hoboken, NJ: Wiley-Blackwell, 2010.
- Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40. [Crossref] [PubMed]
- Bartlett DL, Ramanthan RK, Ben-Josef E. Cancer of the Biliary Tree. In: DeVita VT, Lawrence TS, Rosenberg SA. editors. Cancer Principles and Practice of Oncology. 8th edition. Philadelphia: Lippincott Williams and Wilkins, 2008:1156-86.
- Eckel F, Schmid RM. CHT in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 2007;96:896-902. [Crossref] [PubMed]
- Valle J., Wasan H, Palmer DH. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Ross JS, Wang K, Javle MM, et al. Comprehensive genomic profiling of biliary tract cancers to reveal tumor-specific differences and frequency of clinical relevant genomic alterations. J Clin Oncol 2015;33:abstr 4009.
- Sauri T, Macarulla T, Cabrera G, et al. Comprehensive profiling of biliary tract cancers (BTC) to reveal molecular heterogeneity with implications for matched targeted therapies (MTT). J Clin Oncol 2016;34:abstr 4085.
- Colecistectomía preventiva en adultos de 35 a 49 años. Guía Clínica. Ministerio de Salud. Gobierno de Chile. Available online: http://web.minsal.cl/portal/url/item/72205a1420599f92e04001011f016d02.pdf
- Rege RV. Asymptomatic Gallstones. In: Bland KI, Sarr MG, Büchler MW, et al. editors. General Surgery: Principles and International Practice. London: Springer, 2009:1035-40.