Combined intrahepatic cholangiocarcinoma and hepatocellular carcinoma
Introduction
The majority of primary hepatic malignancies may be classified as either hepatocellular carcinoma (HCC), or intrahepatic cholangiocarcinoma (ICC), each with distinct clinicopathological features. Combined intrahepatic cholangiocarcinoma and hepatocellular carcinoma (cHCC-ICC) is so-called due to histologic evidence of both hepatocellular and biliary epithelial differentiation (1). It is a rare subtype of primary liver cancer, accounting for 1% to 14.2% of cases (1-5). The true incidence may in fact be higher, owing to frequent difficulty in accurate pathological assessment and the advanced nature of many of these tumors on presentation, precluding biopsy. This unique entity was first described by Allen and Lisa in 1949 and has been defined as the intimate intermingling of both a HCC component and CC component (2). Two histopathological classification schemes have been developed (2,6) (Table 1). Molecular analysis in one small case series demonstrated that cHCC-ICC is genetically closer to CC than HCC (7). Due to its rarity, much of the literature to date has been limited to small case series and isolated case reports. Consequently, there is a lack of understanding of the clinicopathological characteristics, prognoses and optimal management of patients with cHCC-ICC.
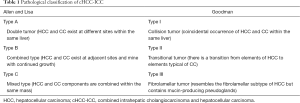
Full table
Epidemiology and risk factors
In terms of HCC as a disease entity, there is clear evidence of geographical variation in incidence which is reflective of variability of risk factors in different populations.
Underlying liver cirrhosis is present in 80% of patients with HCC. Common etiologic factors of hepatic cirrhosis include hepatitis C virus (HCV), hepatitis B virus (HBV), alcohol misuse and non-alcoholic steatohepatitis (8-11). HBV infection is highly prevalent in Africa and Asia (11). HCV is the major risk factor for the development of HCC in Japan, Europe and North America (12). Alcohol misuse and non-alcoholic steatohepatitis, related to obesity and diabetes mellitus, are more commonly implicated in Western populations.
It is likely that such differences also exist for patients with cHCC-ICC, with varying prevalence of underlying hepatic cirrhosis between Eastern and Western populations. Most published series exploring cHCC-ICC originate from Asia and in these, the likelihood of viral hepatitis or liver cirrhosis in patients with cHCC-CC was intermediate between the high proportion seen in patients with HCC and the lower prevalence in patients with CC (3,5,13). In Asia, patients with cHCC-ICC are predominantly male. The prevalence for positive HBV and HCV serologies range from 17–58% and 0–75%, respectively. Underlying liver cirrhosis is present in 23.1–77.8% of cases (14). Taguchi et al. (5) reported that approximately 40% of cHCC-ICC patients in Japan presented with associated cirrhosis and a positive hepatitis serology.
In some studies, cHCC-CC seemed to be a variant of conventional HCC with cholangiocellular features and no significant differences regarding etiological risk factors among patients with cHCC-ICC and HCC were found (13,15,16). In general, patients with cHCC-ICC showed similar clinical and pathological features to HCC patients, including a mean age of onset in the sixth decade, male predominance, a high incidence of HBV infection, underlying chronic liver disease and elevated serum a-fetoprotein (AFP) levels (17).
In contrast, a study by Jarnagin et al. (1) reviewing a cohort of cHCC-ICC from the Western population reported a low prevalence of 15% of positive serology for either HBV or HCV, and complete absence of underlying chronic liver disease, resembling that in the ICC group.
However, given the rising incidence of HCV- and HBV-related HCC in the west, it is conceivable that the proportion of HCV- and HBV-related cHCC-ICC may also increase.
Based on a study of more than 20,000 patients with primary hepatic tumors recorded within SEER (Surveillance, Epidemiology and End Results Program of the National Cancer Institute), cHCC-ICC had an overall incidence of 1.3%; the age and sex specific incidence and geographic variation mirrored those of HCC (18).
Because cHCC-ICC is a tumor in which both HCC and CC components coexist, both risk factors for HCC and oncogenic agents for CC may predispose to cHCC-ICC. Previous studies have included patients with cHCC-ICC with documented risk factors that predispose to CC (1). The exact cause of CC is unknown but there are several well-defined risk factors, the most common of which is primary sclerosing cholangitis (PSC). The true incidence of CC in the setting of PSC is reported as 8–40% (19). Other abnormalities of biliary anatomy that are etiological factors for CC include choledochal cysts and Caroli’s disease, a congenital condition (20,21). Multiple toxic and environmental factors implicated in the pathogenesis of CC may also predispose to cHCC-ICC including dioxin exposure, liver flukes, Thorotrast dye and dietary nitrosamines (22).
Clinical presentation and diagnosis
Typically cHCC-ICC is clinically silent, as is the case with both HCC and CC, until it presents with advanced disease. Common symptoms include painless obstructive jaundice, fatigue, abdominal discomfort, weight loss, pruritus, ascites, acute cholangitis, fever, hepatomegaly and a palpable gallbladder.
Due to striking similarities in the clinical features of cHCC-ICC to those of both conventional HCC and CC, preoperative diagnosis of cHCC-ICC with standard imaging is virtually impossible.
The vast majority of studied patients were initially misdiagnosed either as HCC or CC despite extensive staging scans (23). The accurate preoperative diagnosis of cHCC-ICC does have important implications in terms of management decisions and prognosis, as the treatment selected may differ from that for HCC or CC, depending on the predominant component of the tumor and any underlying cirrhosis. In particular, the frequency of lymph node metastases in cHCC-ICC may be as high as that in CC, underscoring the need for systemic nodal dissection as part of curative surgery (4).
Serologic tumor markers are also of limited benefit for preoperative diagnosis of cHCC-ICC because high levels of serum AFP and low levels of carcinoembryonic antigen (CEA) and/or carbohydrate antigen 19-9 (CA19-9) are most commonly detected (24). Also, previous research has shown that there is no significant difference in AFP levels between the cHCC-ICC group and the HCC group, although a simultaneous increase in serum CA19-9 and AFP plus a liver mass on imaging studies may suggest cHCC-CC (25).
When selecting patients for appropriate treatments, radiologic studies assist in detecting the presence of underlying cirrhosis, extensive intra-hepatic disease, vascular involvement, and extra-hepatic metastases.
There is no characteristic description per se of cHCC-CC on the different imaging modalities. On ultrasound cHCC-ICC usually appears as a hypoechoic mass with a hyperechoic component (26). Computed tomography (CT), positron emission tomography (PET), and fusion PET CT have all been employed to diagnose cHCC-ICC, with varying results. As the histological type of hepatic tumor influences its enhancement characteristics on dynamic CT, findings for cHCC-ICC differ based on the histological proportion of the HCC and CC components and the volume of fibrous stroma (24,27-29) (Table 2).
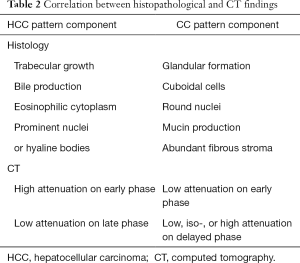
Full table
Dynamic imaging techniques [CT, magnetic resonance imaging (MRI)] exploit the characteristic vascular profile of HCC, which is almost exclusively arterial. cHCC-ICC lesions resembling HCC are identified by intense contrast uptake during the arterial/early phase, whereas there is washout of contrast during the delayed/venous phase (30-33). Contrary to this, in the case of cHCC-ICC lesions resembling CC there is evidence of low attenuation in the early phase, and low, iso- or high attenuation in the delayed phase (31-33). Moreover, in one study it was observed that, in the CC type of cHCC-ICC, CT findings mirrored those of CC but virus markers and serum AFP levels were almost equivalent to those found in patients with a HCC type of cHCC-ICC (29). Thus, cHCC-ICC with a CC pattern component can, in select patients, be diagnosed using the combination of CT findings, serum AFP levels and serum virus markers.
PET can reliably detect CC as small as 1 cm (34), but its detection rate for cHCC-ICC is only about 57% (35). However, fusion PET CT has demonstrated utility in identifying extrahepatic metastases in both HCC and cHCC-ICC patients, with detection rates as high as 98% (35).
Definitive diagnosis of cHCC-ICC on the basis of cytological material alone is difficult, if not impossible, because of a lack of uniform agreement on the cytological criteria for cHCC-ICC. A needle core biopsy is the preferred method for tissue sampling, as it allows the pathologist not only to diagnose malignancy but also to evaluate the architecture/pattern of the tumor, which is critical for accurate diagnosis of cHCC-ICC. Needle core biopsies are not without their limitations however, as given the diverse appearance of these tumors and inherent heterogeneity, the material biopsied may contain only one of the two components resulting in a misdiagnosis of either HCC or CC. The diagnostic yield of biopsy for pre-operative diagnosis of cHCC-ICC is low as reported by Taguchi et al. (5); none of the 23 patients with cHCC-ICC showed features of cHCC-ICC on preoperative liver biopsies, 20 out of 23 had HCC on biopsy and the other three were diagnosed with CC on biopsy. Final diagnosis of this entity is usually made on surgical specimens or liver explant.
Identification and classification
Classification of cHC-ICC has been an evolving field over several decades. The first true detailed characterization of cHCC-ICC as an individual entity was in 1949 by Allen and Lisa who reported that the combined tumor accounted for approximately 1.4% of all primary hepatic malignancies (2). In 1989, The Liver Cancer Study Group of Japan formulated its own classification of cHCC-CC (4). According to this system, cHCC-CC was again classified into three types: double cancer, combined type, and mixed type. In 1985, Goodman et al., while reporting 24 cases of cHCC-ICC, suggested a third new system of classification: collision, transitional and fibrolamellar tumors (6).
According to the World Health Organization (WHO), cHCC-ICC is defined as a tumor with an intimate and unequivocal admixture of both HCC and ICC cells (36). This category should not be used for tumors in which either form of growth is insufficiently differentiated for positive identification. The WHO defines the hepatocellular component of cHCC-ICC by the presence of bile production, bile canaliculi and a trabecular pattern of growth. The glandular component is defined by the presence of true gland formation with mucin production. Current WHO guidelines classify cHCC-ICC into two subtypes: cHCC-ICC classical type and cHCC-ICC with stem cell features. The latter type is extremely rare and is further subcategorized into the following three subtypes: typical, intermediate cell and cholangiocellular subtype. The most common form of cHCC-ICC is the classical type. It contains areas of typical appearing HCC intermixed with CC and identifiable transition zones, where the two components merge and show tumor cells with intermediate morphology.
The variation in the reported incidence of cHCC-ICC has been attributed in part to lack of uniform criterion for diagnosis and classification of these tumors. Some reports include only cHCC-ICC of Allen type C (1,15) whereas, others incorporate all three types of cHCC-ICC (5,16,37).
The diagnosis of cHCC-ICC is reliant on unequivocal evidence of both hepatocellular and biliary epithelial features within the same tumor. Features suggestive of HCC differentiation include trabecular or pseudoglandular growth pattern, bile in the canaliculi, and carcinoma cells with intracellular inclusions such as fat, Mallory bodies and alpha-1 antitrypsin typically found in hepatocytes. Features resembling CC include a desmoplastic stroma, and carcinoma cells forming glandular structures and less commonly, producing mucin. A solid diagnosis of cHCC-ICC mandates an interface where HCC and CC components intimately intermingle with each other (24). Classical histological features of cHCC-ICC are shown in Figure 1. Notably, however, some tumors may show varying degrees of hepatocellular and cholangiocytic differentiation without a definable interface, and tumor classification can thus be challenging.
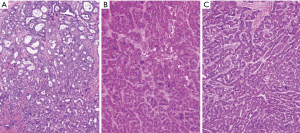
Immunohistochemistry plays a key role in confirming the individual components of the tumor as well as demonstrating transition zones. Confirmation of a HCC element is provided by immunohistochemical staining with hepatocellular markers [including hepatocyte paraffin 1 (HepPar-1) monoclonal antibody and Arginase 1], canalicular staining with polyclonal CEA or CD10 and demonstration of sinusoidal “capillarization” by CD34. The CC component is diagnosed by positive immunohistochemical staining for CK7, CK19 and MOC31, and luminal reactivity to epithelial membrane antigent (EMA) (38-40). A mucin stain [periodic acid-Schiff stain after diastase digestion (dPAS) or mucicarmine] may also aid in demonstrating the CC component by highlighting intracytoplasmic mucin (36). Transition zones have been shown to stain for both typical biliary cytokeratins CK7 and CK19, as well as the hepatocellular markers HepPar-1 and Arginase 1. Recently, branched chain in situ hybridization for albumin has been shown to be an additional tool sensitive in detecting epithelial tumors of hepatic origin including both HCC and CC (41).
The cell of origin in cHCC-ICC remains unknown. Studies have shown that HCC, CC and many other tumors may originate from stem cells (42-44). It is generally accepted that adult hepatic stem cells are the hepatic oval cells and they are capable of biliary or hepatocytic differentiation (43,44).
In cases of cHCC-ICC, it has been observed that the tumor is derived from a single clone, and the histological diversity is a phenotypic expression of divergent differentiation (45). Yano et al. (46) established a primary cell line derived from resected cHCC-ICC and showed that it differentiated to not only the characteristics of HCC but also those of CC under different growth conditions, suggesting that cHCC-ICC arise from stem cells. Woo et al. (47) investigating the comparative gene expression profile of ICC, HCC and cHCC-ICC tumors observed that ICC and HCC could be clearly distinguished from one another by their gene expression profile. Further, 5/7 (71.4%) cHCC-ICC tumors clustered together with the ICC group, suggesting that cHCC-ICC is closer to ICC (than HCC) at the molecular level.
Treatment approaches
There are no clear guidelines with regard to the management of cHCC-ICC. However, surgical resection is the only treatment offering the possibility of a cure. Factors which influence eligibility for surgery include underlying cirrhosis, the patient’s general medical condition, tumor extent and local anatomic conditions. The main goal should be complete surgical excision with negative margins and limited impingement on liver function. Severe liver dysfunction predicts a dismal prognosis, irrespective of the technical success of the procedure.
cHCC-ICC tends to behave like HCC with respect to portal and hepatic venous infiltration, and like CC with regard to lymph node metastasis (6,48). At autopsy, lymph node metastasis was observed in 76% of patients with cHCC-ICC (4). Compared with this, lymph node metastasis was present in only 30% of HCC patients and 69% of CC patients (4). Consequently, hepatic resection with hilar lymph node dissection is the recommended treatment for cHCC-ICC in non-cirrhotic patients. Resectability in this group has been reported to be as high as 78% (1). However, the prognostic benefit of lymphadenectomy for cHCC-CC remains controversial (49-52). Whether the addition of systemic chemotherapy in a neoadjuvant or adjuvant setting in patients undergoing node dissection improves prognosis also remains an open question.
For cirrhotic patients, hepatic resection has the potential for debilitating complications (11), so the adoption of strict selection criteria is imperative to negate significant peri-operative as well as overall morbidity and mortality. The reduced functional reserve of cirrhotic patients limits the extent of surgery.
The role of liver transplantation in the management of HCC is well accepted and defined. Liver transplantation is an effective option for patients with HCC and cirrhosis corresponding to the Milan criteria (53), i.e., solitary tumors of <5 cm or up to three tumor nodules each of which is smaller than 3 cm. Because of the high rate of tumor recurrence and a lack of positive prognostic variables, liver transplantation as a treatment for CC is generally considered to be contra-indicated (54,55).
Data about the role of liver transplantation in the management of cHCC-ICC are lacking. In a study of three cHCC-ICC patients treated by liver transplantation, two patients survived at 25 and 35 months after liver transplantation respectively (56). In this study, the survival of these patients surpassed that of patients who underwent resection; however, the small size of the study makes any conclusions unreliable, and more research is required. Panjala et al. (57) recently reported that among patients who underwent liver transplant for HCC, 12 had mixed cHCC-ICC and ICC in the explant. The tumor recurrence rate was 58%, and the 5-year overall patient survival rate was 16% in cHCC-ICC patients who underwent liver transplantation. Sapisochin et al. (58) reported survival data on a similar 14 patients who were found to have cHCC-ICC on the explant. Eight of the 14 patients (57%) had tumor recurrence after a median follow-up of 32 months. The median disease-free survival time was 8 months. The cumulative risk of tumor recurrence was 40% and 70% at 1 and 5 years, respectively, for those 14 patients. There are no published reports describing non-surgical treatment options for cHCC-ICC.
Transarterial chemoembolisation (TACE) and percutaneous treatments such as percutaneous ethanol injection (PEI) and radiofrequency ablation (RFA) are the most widely used treatments for unresectable HCC and in the setting of post-resection recurrence (59,60). However, many cHCC-ICC tumors are less vascular and more fibrotic than HCC, and thus are less likely to respond to either TACE or PEI.
Other approaches, such as RFA or cryoablation, may be of benefit for treatment of recurrence in select patients (61). The pattern of local recurrence of cHCC-ICC is thought to reflect that of CC rather than HCC, indicating the dominant prognostic role of the CC element of cHCC-ICC (23,48). Therefore, patients who are unresectable due to locally advanced disease or local recurrence in the absence of distant metastases may be candidates for palliative radiation therapy with or without concomitant chemotherapy. Symptomatic and local tumor control have been reported with such a treatment paradigm (6,62,63).For those patients with widespread metastatic disease, systemic chemotherapy may be an option for fit patients; however, the reported response rates are low (64-66).
Survival
Survival is determined by disease stage at diagnosis and, perhaps, the absence of nodal involvement. Partial hepatectomy with hilar lymph node dissection can result in 5-year survival rates exceeding 50% in patients with early stage disease (23,50). Post-surgical resection, the reported median length of survival ranges from 20 to 47 months (1,3,15,24,39,67).
Vascular and lymph node invasion and the presence of satellite metastases, which are related to and associated with tumor size, have been suggested as significant predictors of poor prognosis after resection (3,15,23,33,68). In contrast, patients with unresectable tumors fared poorly, with no survivors reported beyond 2 years (1,39).
In a small retrospective study of 25 patients (17), multivariate analysis showed that elevated CA 19-9 (>/= 80 U⁄mL) and the presence of intrahepatic biliary dilatation were independent predictors of poor survival. In general, the survival rate of cHCC-ICC was either lower than that reported for HCC or CC (1,13-14,67) or intermediate between HCC and CC (3,17,23). Lower survival after surgical resection of cHCC-ICC is likely related to the lack of effective treatment options for disease recurrence, which is as high as 95% within the first 2 years (13,15,33).
Conclusions
cHCC-ICC is an uncommon primary liver neoplasm which is increasingly being recognized as a distinct entity. Its true incidence may be higher than that reported due to historical diagnostic difficulties in distinguishing between patients with ICC, HCC and those with cHCC-ICC. The clinical behavior and prognosis of cHCC-CC has overlapping features between that of HCC and ICC. Pre-operative diagnosis of cHCC-CC is crucial but remains difficult. Successful preoperative diagnosis is dependent upon maintaining a high index of suspicion, appropriate selection of imaging studies, obtaining histology if possible, and correlating the results with serum AFP and CA 19-9. As cHCC-ICC is a rare disease entity, no clear treatment paradigm has yet been defined. Currently, surgery remains the only effective treatment option. In those eligible for surgery, the recommended approach is partial hepatectomy with hilar lymph node dissection. The exact role of liver transplantation remains unclear and requires further investigation. These diagnostic and therapeutic dilemmas, which in part relate to the origin of cHCC-ICC from two different tumor entities, combined with the inherent poor prognosis of cHCC-ICC highlight the need for increased effort in understanding the pathogenesis to improve diagnosis and ultimately treatment outcomes for patients with this malignancy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jarnagin WR, Weber S, Tickoo SK, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer 2002;94:2040-6. [Crossref] [PubMed]
- Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol 1949;25:647-55. [PubMed]
- Koh KC, Lee H, Choi MS, et al. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg 2005;189:120-5. [Crossref] [PubMed]
- Liver Cancer Study Group of Japan. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg 1990;211:277-87. [PubMed]
- Taguchi J, Nakashima O, Tanaka M, et al. A clinicopathological study on combined hepatocellular and cholangiocarcinoma. J Gastroenterol Hepatol 1996;11:758-64. [Crossref] [PubMed]
- Goodman ZD, Ishak KG, Langloss JM, et al. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer 1985;55:124-35. [Crossref] [PubMed]
- Cazals-Hatem D, Rebouissou S, Bioulac-Sage P, et al. Clinical and molecular analysis of combined hepatocellular-cholangiocarcinomas. J Hepatol 2004;41:292-8. [Crossref] [PubMed]
- Beasley RP, Hwang LY, Lin CC, et al. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet 1981;2:1129-33. [Crossref] [PubMed]
- Colombo M, Kuo G, Choo QL, Donato MF, et al. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet 1989;2:1006-8. [Crossref] [PubMed]
- Sun Z, Lu P, Gail MH, et al. Increased risk of hepatocellular carcinoma in male hepatitis B surface antigen carriers with chronic hepatitis who have detectable urinary aflatoxin metabolite M1. Hepatology 1999;30:379-83. [Crossref] [PubMed]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17. [Crossref] [PubMed]
- Bruix J, Boix L, Sala M, et al. Focus on hepatocellular carcinoma. Cancer Cell 2004;5:215-9. [Crossref] [PubMed]
- Liu CL, Fan ST, Lo CM, et al. Hepatic resection for combined hepatocellular and cholangiocarcinoma. Arch Surg 2003;138:86-90. [Crossref] [PubMed]
- Zhou YM, Yang JM, Wang B, et al. Combined hepatocellular carcinoma and cholangiocarcinoma: a case report and review of the literature. Hepatobiliary Pancreat Dis Int 2007;6:656-9. [PubMed]
- Yano Y, Yamamoto J, Kosuge T, et al. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol 2003;33:283-7. [Crossref] [PubMed]
- Ng IO, Shek TW, Nicholls J, et al. Combined hepatocellular-cholangiocarcinoma: a clinicopathological study. J Gastroenterol Hepatol 1998;13:34-40. [Crossref] [PubMed]
- Chantajitr S, Wilasrusmee C, Lertsitichai P, et al. Combined hepatocellular and cholangiocarcinoma: clinical features and prognostic study in a Thai population. J Hepatobiliary Pancreat Surg 2006;13:537-42. [Crossref] [PubMed]
- Wachtel MS, Zhang Y, Xu T, et al. Combined hepatocellular cholangiocarcinomas; analysis of a large database. Clin Med Pathol 2008;1:43-7. [PubMed]
- Anderson CD, Pinson CW, Berlin J, et al. Diagnosis and treatment of cholangiocarcinoma. Oncologist 2004;9:43-57. [Crossref] [PubMed]
- Kassahun WT, Kahn T, Wittekind C, et al. Caroli's disease: liver resection and liver transplantation. Experience in 33 patients. Surgery 2005;138:888-98. [Crossref] [PubMed]
- Lipsett PA, Pitt HA, Colombani PM, et al. Choledochal cyst disease. A changing pattern of presentation. Ann Surg 1994;220:644-52. [Crossref] [PubMed]
- Pitt HA, Dooley WC, Yeo CJ, et al. Malignancies of the biliary tree. Curr Probl Surg 1995;32:1-90. [Crossref] [PubMed]
- Tang D, Nagano H, Nakamura M, et al. Clinical and pathological features of Allen's type C classification of resected combined hepatocellular and cholangiocarcinoma: a comparative study with hepatocellular carcinoma and cholangiocellular carcinoma. J Gastrointest Surg 2006;10:987-98. [Crossref] [PubMed]
- Maeda T, Adachi E, Kajiyama K, et al. Combined hepatocellular and cholangiocarcinoma: proposed criteria according to cytokeratin expression and analysis of clinicopathologic features. Hum Pathol 1995;26:956-64. [Crossref] [PubMed]
- Kassahun WT, Hauss J. Management of combined hepatocellular and cholangiocarcinoma. Int J Clin Pract 2008;62:1271-8. [Crossref] [PubMed]
- Choi BI, Han JK, Kim YI, et al. Combined hepatocellular and cholangiocarcinoma of the liver: sonography, CT, angiography, and iodized-oil CT with pathologic correlation. Abdom Imaging 1994;19:43-6. [Crossref] [PubMed]
- Araki T, Itai Y, Furui S, et al. Dynamic CT densitometry of hepatic tumors. AJR Am J Roentgenol 1980;135:1037-43. [Crossref] [PubMed]
- Gibson JB. Histological Typing of Tumors of the Liver, Biliary Tract, and Pancreas. Geneva, Switzerland: World Health Organization, 1978.
- Asayama Y, Taguchi Ki K, Aishima Si S, et al. The mode of tumour progression in combined hepatocellular carcinoma and cholangiocarcinoma: an immunohistochemical analysis of E-cadherin, alpha-catenin and beta-catenin. Liver 2002;22:43-50. [Crossref] [PubMed]
- Honda H, Ochiai K, Adachi E, et al. Hepatocellular carcinoma: correlation of CT, angiographic, and histopathologic findings. Radiology 1993;189:857-62. [Crossref] [PubMed]
- Honda H, Onitsuka H, Yasumori K, et al. Intrahepatic peripheral cholangiocarcinoma: two-phased dynamic incremental CT and pathologic correlation. J Comput Assist Tomogr 1993;17:397-402. [Crossref] [PubMed]
- Fukukura Y, Taguchi J, Nakashima O, et al. Combined hepatocellular and cholangiocarcinoma: correlation between CT findings and clinicopathological features. J Comput Assist Tomogr 1997;21:52-8. [Crossref] [PubMed]
- Sanada Y, Shiozaki S, Aoki H, et al. A clinical study of 11 cases of combined hepatocellular-cholangiocarcinoma Assessment of enhancement patterns on dynamics computed tomography before resection. Hepatol Res 2005;32:185-95. [Crossref] [PubMed]
- Anderson CD, Rice MH, Pinson CW, et al. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg 2004;8:90-7. [Crossref] [PubMed]
- Nagaoka S, Itano S, Ishibashi M, et al. Value of fusing PET plus CT images in hepatocellular carcinoma and combined hepatocellular and cholangiocarcinoma patients with extrahepatic metastases: preliminary findings. Liver Int 2006;26:781-8. [Crossref] [PubMed]
- Theise ND, Nakashima O, Park YN, et al. Combined hepatocellular-cholangiocarcinoma. In: Bosman FT, Carneiro F, Hruban RH, et al. editors. WHO classiification of tumors of the digestive system. Lyon: IARC Press, 2010:225-7.
- Aoki K, Takayasu K, Kawano T, et al. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features and computed tomographic findings. Hepatology 1993;18:1090-5. [Crossref] [PubMed]
- Leong AS, Sormunen RT, Tsui WM, et al. Hep Par 1 and selected antibodies in the immunohistological distinction of hepatocellular carcinoma from cholangiocarcinoma, combined tumours and metastatic carcinoma. Histopathology 1998;33:318-24. [Crossref] [PubMed]
- Tickoo SK, Zee SY, Obiekwe S, et al. Combined hepatocellular-cholangiocarcinoma: a histopathologic, immunohistochemical, and in situ hybridization study. Am J Surg Pathol 2002;26:989-97. [Crossref] [PubMed]
- Kondo F, Fukusato T. Pathogenesis of Cholangiolocellular Carcinoma: Possibility of an Interlobular Duct Origin. Intern Med 2015;54:1685-94. [Crossref] [PubMed]
- Shahid M, Mubeen A, Tse J, et al. Branched chain in situ hybridization for albumin as a marker of hepatocellular differentiation: evaluation of manual and automated in situ hybridization platforms. Am J Surg Pathol 2015;39:25-34. [Crossref] [PubMed]
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105-11. [Crossref] [PubMed]
- Alison MR. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Rev 2005;1:253-60. [Crossref] [PubMed]
- Wu XZ, Chen D. Origin of hepatocellular carcinoma: role of stem cells. J Gastroenterol Hepatol 2006;21:1093-8. [Crossref] [PubMed]
- Fujii H, Zhu XG, Matsumoto T, et al. Genetic classification of combined hepatocellular-cholangiocarcinoma. Hum Pathol 2000;31:1011-7. [Crossref] [PubMed]
- Yano H, Iemura A, Haramaki M, et al. A human combined hepatocellular and cholangiocarcinoma cell line (KMCH-2) that shows the features of hepatocellular carcinoma or cholangiocarcinoma under different growth conditions. J Hepatol 1996;24:413-22. [Crossref] [PubMed]
- Woo HG, Lee JH, Yoon JH, et al. Identification of a cholangiocarcinoma-like gene expression trait in hepatocellular carcinoma. Cancer Res 2010;70:3034-41. [Crossref] [PubMed]
- Uenishi T, Hirohashi K, Shuto T, et al. Surgery for mixed hepatocellular and cholangiocellular carcinoma. Hepatogastroenterology 2000;47:832-4. [PubMed]
- Nakamura S, Suzuki S, Sakaguchi T, et al. Surgical treatment of patients with mixed hepatocellular carcinoma and cholangiocarcinoma. Cancer 1996;78:1671-6. [Crossref] [PubMed]
- Sasaki A, Kawano K, Aramaki M, et al. Clinicopathologic study of mixed hepatocellular and cholangiocellular carcinoma: modes of spreading and choice of surgical treatment by reference to macroscopic type. J Surg Oncol 2001;76:37-46. [Crossref] [PubMed]
- Vauthey JN, Pawlik TM, Abdalla EK, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg 2004;239:722-30; discussion 730-2. [Crossref] [PubMed]
- Ercolani G, Grazi GL, Ravaioli M, et al. The role of lymphadenectomy for liver tumors: further considerations on the appropriateness of treatment strategy. Ann Surg 2004;239:202-9. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation 2000;69:1633-7. [Crossref] [PubMed]
- Shimoda M, Farmer DG, Colquhoun SD, et al. Liver transplantation for cholangiocellular carcinoma: analysis of a single-center experience and review of the literature. Liver Transpl 2001;7:1023-33. [Crossref] [PubMed]
- Chan AC, Lo CM, Ng IO, et al. Liver transplantation for combined hepatocellular cholangiocarcinoma. Asian J Surg 2007;30:143-6. [Crossref] [PubMed]
- Panjala C, Senecal DL, Bridges MD, et al. The diagnostic conundrum and liver transplantation outcome for combined hepatocellular-cholangiocarcinoma. Am J Transplant 2010;10:1263-7. [Crossref] [PubMed]
- Sapisochin G, Fidelman N, Roberts JP, et al. Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transpl 2011;17:934-42. [Crossref] [PubMed]
- Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. [Crossref] [PubMed]
- Okada S. Local ablation therapy for hepatocellular carcinoma. Semin Liver Dis 1999;19:323-8. [Crossref] [PubMed]
- Dick EA, Taylor-Robinson SD, Thomas HC, et al. Ablative therapy for liver tumours. Gut 2002;50:733-9. [Crossref] [PubMed]
- Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology 2004;66:167-79. [Crossref] [PubMed]
- Todoroki T, Ohara K, Kawamoto T, et al. Benefits of adjuvant radiotherapy after radical resection of locally advanced main hepatic duct carcinoma. Int J Radiat Oncol Biol Phys 2000;46:581-7. [Crossref] [PubMed]
- Kajanti M, Pyrhönen S. Epirubicin-sequential methotrexate-5-fluorouracil-leucovorin treatment in advanced cancer of the extrahepatic biliary system. A phase II study. Am J Clin Oncol 1994;17:223-6. [Crossref] [PubMed]
- Takada T, Kato H, Matsushiro T, et al. Comparison of 5-fluorouracil, doxorubicin and mitomycin C with 5-fluorouracil alone in the treatment of pancreatic-biliary carcinomas. Oncology 1994;51:396-400. [Crossref] [PubMed]
- Connell LC, Harding JJ, Lowery MA, et al. Platinum-based combination therapy (PCT) and outcomes for patients (pts) with mixed hepatocellular carcinoma and intrahepatic cholangiocarcinoma (mHCC/ICC). J Clin Oncol 2015;33:abstr e15146.
- Lee WS, Lee KW, Heo JS, et al. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Today 2006;36:892-7. [Crossref] [PubMed]
- Shin CI, Lee JM, Kim SH, et al. Recurrence patterns of combined hepatocellular-cholangiocarcinoma on enhanced computed tomography. J Comput Assist Tomogr 2007;31:109-15. [Crossref] [PubMed]


