Surgical management of biliary tract cancers
Introduction
Complete resection remains the only potentially curative therapy for biliary tract cancers (BTC). Unfortunately, patients most commonly present with unresectable or metastatic disease, and recurrence rates remain high after complete resection. The overall prognosis for patients with BTC is poor with 5-year overall survival rates as low as 5–15% (1,2). This review focuses on the current surgical strategies in the management of BTCs including gallbladder cancer, intrahepatic, and extrahepatic cholangiocarcinoma.
Gallbladder cancer
Clinical presentation
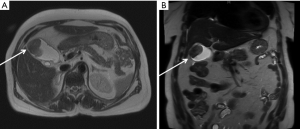
Gallbladder cancer typically presents one of three ways: (I) suspicion of malignancy preoperatively; (II) malignancy suspected intra-operatively; and (III) malignancy diagnosed incidentally following cholecystectomy. Incidental diagnosis of gallbladder cancer on final pathology following a cholecystectomy for suspected benign biliary disease is the most common presentation and is reported to occur following 0.25% of laparoscopic cholecystectomies (3). Duffy et al. reported their experience from Memorial Sloan Kettering Cancer Center (MSKCC) in which nearly half (47%) of the 435 gallbladder cancer cases were diagnosed incidentally following a cholecystectomy (4). Early stage gallbladder cancer is commonly asymptomatic. When symptomatic, abdominal pain is the most common symptom followed by jaundice and weight loss, which are typically signs of advanced disease. A gallbladder mass may occasionally be found during workup of suspected benign biliary disease (Figure 1) (1,3). Jaundice at the time of presentation is a particularly ominous sign and has been shown to be an indicator of advanced disease and these patients are unlikely to benefit from surgical resection. A study investigating the clinical significance of jaundice in patients with gallbladder cancer by Hawkins et al. found that of the 82 patients with jaundice, 79 (96%) had advanced-stage (III and IV) disease with a median disease-specific survival of 6 months compared to 16 months when jaundice was absent (P=0.0001) (5).
Surgical management
A macroscopically complete surgical resection with negative microscopic margins (R0) remains the only potentially curative treatment for gallbladder cancer. The surgical approach is often influenced by the circumstances of diagnosis. The majority of cases will be discovered incidentally after a cholecystectomy and tumor staging will then determine what, if any, further resection is indicated. Thorough preoperative assessment is imperative. The operative note should be reviewed or direct communication with the referring surgeon should take place to completely understand the initial operative findings. Pathology slides should be obtained and re-reviewed to determine the exact T-stage, radial margin, and cystic duct margin. Additionally, the presence of metastatic disease should be ruled out by updated cross-sectional imaging including the chest, abdomen and pelvis. Outcomes, compared stage for stage, have not been shown to be different in patients who undergo upfront primary curative resection or initial cholecystectomy followed by definitive resection. Fong et al. demonstrated that the survival of 80 patients who underwent an initial non-curative cholecystectomy followed by definitive resection was no different than 22 patients who had an initial definitive resection (6). Shih et al. similarly found no significant difference in survival in 53 patients who underwent resection during the original operation compared to those that were resected at a separate setting after cholecystectomy (7). However, these reports must be interpreted with caution, given the significant selection bias among the patients who ultimately undergo definitive resection after cholecystectomy. When a definitive operation is undertaken, the T-stage determined at the time of cholecystectomy dictates the extent of the resection (Table 1).
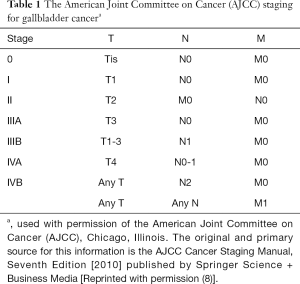
Full table
T1a
T1a tumors are confined to the lamina propria and are most commonly an incidental finding following cholecystectomy. A simple cholecystectomy alone has been accepted as the gold standard for these patients as long as the margins, including the cystic duct, are negative. As the dissection during a cholecystectomy is done within the perimuscular layer, and by definition, T1a tumors have not invaded the muscularis layer; a simple cholecystectomy is the definitive operation. Contemporary series report the 5-year survival following simple cholecystectomy for T1a gallbladder cancer to be excellent ranging 97–99% (9,10). Survival rates this high make it difficult to argue for a more extensive resection. Recurrence rates for T1a tumors are low and have been reported to range 0.6–3.4% (9,10).
T1b
T1b tumors invade into the muscular layer and have been the topic of debate regarding the appropriate extent of resection. Several studies have advocated that a simple cholecystectomy alone is an adequate approach for T1b tumors (11-14). However, other groups have shown that patients with T1b tumors are at a higher risk for recurrence given the risk of residual disease in the gallbladder fossa after simple cholecystectomy (15-17). Rates of residual hepatic disease in patients with T1b tumors have been reported in up to 10% of patients, with rates of lymph node positivity of up to 15% (18). It is currently the recommendation of the National Comprehensive Cancer Network (NCCN) as well as the American Hepato-Pancreato-Biliary Association that patients with T1b gallbladder cancer undergo an extended cholecystectomy with en bloc resection of adjacent liver parenchyma to include segments IVb and V, in addition to a regional lymph node dissection (19,20). Bile duct resection should only be performed if the cystic duct margin is positive necessitating a wider margin. Elective bile duct resection (when not necessary to achieve R0 resection) has not been associated with a survival benefit or improved nodal yield (21,22).
T2
T2 tumors extend through the perimuscular connective tissue and are associated with a high rate of nodal positivity, which is seen in 39–46% of patients (23,24). The 5-year overall survival rates for patients with T2 tumors undergoing simple cholecystectomy and extended cholecystectomy is 17–38% and 59–90%, respectively (12,24-26). The recommended operative approach is identical to that of T1b tumors—hepatic resection including segments IVb and V with a regional lymphadenectomy of the hepatoduodenal ligament. If the cystic duct margin is positive for cancer on the original assessment, re-excision of the cystic duct or a bile duct resection to achieve a negative margin is required.
T3 and T4
While T3 tumors penetrate the gallbladder serosa and/or invade the liver or an adjacent organ, T4 tumors invade two or more adjacent organs or invade the main portal vein or hepatic artery. These locally advanced gallbladder tumors create a challenge for surgeons in order to balance the morbidity of an extensive operation with a possible survival benefit. Historically, extensive resection was considered futile in this group. Resections in this group of patients depend on the extent of tumor but can require major hepatic resections (extended right hepatectomy) or bile duct resection to obtain negative margins. These operations are associated with significant morbidity and more limited resections, whenever possible, should be attempted. In a series from France, 85% of the 724 patients included had T3/T4 tumors with a reported median survival of 3–6 months (27). However, more recently, more favorable results have been reported for both T3 and T4 tumors (25,28,29). In a study from MSKCC that included 63 patients with T3/T4 tumors who underwent resection, the 5-year overall survival was reported to be 25%, with nearly all of these long-term survivors having N0 disease (up to 26% of these cases) (22). These results demonstrate a potential survival benefit to resection; however, they apply to a select subgroup of patients who have locally advanced disease without evidence of lymph node metastases outside the regional lymph node basin or distant metastatic disease.
Extent of resection for pre- or intra-operative suspicion for gallbladder cancer
When gallbladder cancer is suspected intra-operatively during cholecystectomy for presumed benign disease, a frozen section should be obtained to confirm the diagnosis. If the operating surgeon is skilled in the area of hepatobiliary surgery, a curative intent operation should be performed—a complete en bloc resection of the gallbladder with segment IVb and V of the liver, and a portal lymphadenectomy. More extensive resection including the right lobe of the liver and bile duct are only necessary to achieve clear margins. If the skill and resources are not available, the patient should be transferred to a specialized hepatobiliary center. If a gallbladder mass is detected on preoperative imaging, and is suspicious for tumor, it is appropriate to proceed to definitive resection understanding the risk that the ultimate pathology may demonstrate benign disease. In general, biopsies of the gallbladder should be avoided because of the propensity of gallbladder cancers to spread to the peritoneum. If there is a suspicion for gallbladder cancer intra-operatively, care must be taken to avoid spillage of bile that could lead to peritoneal tumor dissemination. Any evidence of peritoneal spread such as nodules or plaques should be biopsied to aid in disease staging.
Bile duct resection
Routine bile duct resection is an area of debate with prior groups reporting conflicting impacts on survival (21,30,31). More recently, D’Angelica et al. used a prospective database to investigate the impact of the extent of resection for gallbladder cancer on morbidity and survival (22). Sixty-eight of the 109 patients (65%) identified underwent resection of the common bile duct, of which 36 patients had gross involvement of the bile duct requiring resection while 32 patients underwent empiric bile duct resection. Bile duct resection had no significant impact on disease-specific survival and was associated with a higher morbidity. While portal lymph node dissection may be facilitated by concomitant bile duct resection, there was no difference in the number of lymph nodes removed between those who underwent bile duct resection and those who did not. Bile duct resection should be reserved for those patients with suspected tumor involvement of the porta hepatis and should not be done empirically.
Lymph node dissection
Portal lymph node dissection is recommended for T1b–T4 tumors and should include porta hepatis, gastrohepatic ligament, and retroduodenal lymph nodes. Celiac, retropancreatic and/or inter-aortocaval lymph nodes should be assessed early in the procedure, as if positive, the procedure should be aborted due to the associated poor prognosis. There is no universally accepted minimum number of lymph nodes that should be removed. The number of lymph nodes removed has, however, been shown to correlate with overall survival (32,33). A cutoff of three lymph nodes has been suggested, whereby less than three nodes removed has been shown to be associated with a worse survival (34). The American Hepato-Pancreato-Biliary Association on the other hand recommends resecting at least six lymph nodes (20). Lymph node dissection provides important prognostic information and may provide regional disease control.
Staging laparoscopy
Staging laparoscopy at the time of a planned resection for gallbladder cancer has the potential of identifying unresectable disease and thus sparing the patient a laparotomy. Weber et al. prospectively reviewed 44 patients at MSKCC with potentially resectable gallbladder cancer and found that the yield of staging laparoscopy was 48%, identifying 21 patients with unresectable disease (35). More recently, Butte et al. reported that only 10 of 48 patients (21.8%) undergoing staging laparoscopy were found to have disseminated disease, concluding that staging laparoscopy has a limited role in gallbladder cancer (36). The authors did find that disseminated disease was present in nearly one-third of patients with T3 tumors and that a positive cholecystectomy margin and poor tumor differentiation were independent factors associated with the likelihood of disseminated disease, suggesting the benefit of staging laparoscopy should be limited to this patient subset.
Port site recurrences
A port site recurrence is a theoretical risk when resecting a malignancy laparoscopically. As the majority of gallbladder cancers are incidentally discovered following a laparoscopic cholecystectomy, the potential of tumor dissemination from bile spillage and port site seeding has been a great concern. While some groups have routinely resected either the site of specimen removal or all port sites, there is no data to support this practice. In a recent study from MSKCC, 113 patients who were incidentally discovered to have gallbladder cancer following laparoscopic cholecystectomy, port site recurrences were seen in 19%, however, port site resection had no association with survival or recurrence (37). Importantly, port site metastases are associated with diffuse peritoneal recurrence and a poor prognosis (38,39).
Cholangiocarcinoma
Clinical presentation
Clinical presentation will vary depending on the location of the tumor. Initial symptoms are commonly nonspecific. Extrahepatic cholangiocarcinoma will typically present with signs and symptoms of biliary obstruction including jaundice, pruritus, dark urine, and pale stool, in addition to weight loss and anorexia. Intrahepatic cholangiocarcinoma is less likely to present with biliary obstruction and often presents as an asymptomatic hepatic mass found incidentally on imaging or in advanced disease with abdominal pain or weight loss. In a series of 294 patients with cholangiocarcinoma, Nakeeb et al. found that approximately 90% of patients with extrahepatic cholangiocarcinoma presented with jaundice while abdominal pain was more common in patients with intrahepatic cholangiocarcinoma (40). Although biliary obstruction is common, cholangitis is an unusual presentation.
Surgical management
Intrahepatic cholangiocarcinoma
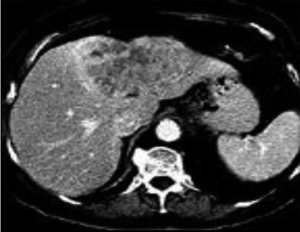
Intrahepatic cholangiocarcinomas arise from within the hepatic parenchyma from the biliary tree proximal to the bifurcation of the left and right hepatic duct (Figure 2). Complete resection with negative microscopic margins is the only treatment that is associated with long-term survival and cure. Five-year overall survival rates after complete resection range from 22–42% (41-43) (Table 2). Unfortunately, two-thirds of patients will present with unresectable disease (44). Contraindications to resection include involvement of inflow or outflow bilaterally, multiple intrahepatic tumors, and metastatic disease to distant sites (Table 3). The goal of hepatic resection is to resect the tumor with a negative margin. While a parenchymal sparing resection is preferred, due to the typically large size at presentation, major liver resection is often required to achieve a negative margin. Endo et al. reviewed the MSKCC experience in which 78% of patients (n=64) undergoing resection of intrahepatic cholangiocarcinoma required a major hepatectomy with extrahepatic bile duct resection and vascular resection (portal vein and/or inferior vena cava) required in 17 patients (21%) and 7 patients (9%), respectively (44). In a study from the Italian Intrahepatic Cholangiocarcinoma Study Group that included 434 patients, R0 resection was associated with improved 5-year overall survival (39.8% vs. 4.7%, P<0.0.001) and lower recurrence rates (53.9% vs. 73.6%, P=0.005) compared to patients with a positive margin (45). The dismal 5-year overall survival in patients with positive margins indicates that resection should only be undertaken with the intent of an R0 resection.

Full table

Full table
The utility of routine lymph node dissection is unclear in patients undergoing resection for intrahepatic cholangiocarcinoma. The presence of lymph node metastases has been shown to be one of the most important prognostic factors (44,46). A recent systematic review by Amini et al. reported that the majority of patients (78.5%) undergo lymph node dissection in addition to hepatic resection, of which 45.2% were found to have lymph node metastasis, which was associated with a 3- and 5-year overall survival 2% and 0%, respectively (47). However, there although there is no data to demonstrate a therapeutic benefit to performing a routine lymph node dissection, due to its prognostic value, lymph node dissection should be considered at the time of resection.
Extrahepatic cholangiocarcinoma
Extrahepatic cholangiocarcinoma is the most common type of cholangiocarcinoma. These tumors are sub-classified into hilar cholangiocarcinomas (Klatskin tumors) which occur at the junction of the right and left hepatic ducts, and distal cholangiocarcinoma which occur in the extrahepatic bile duct proximal to the ampulla of Vater. Hilar cholangiocarcinoma is the most common subtype and account for 40–60% of all cases. Complete resection provides the only potential curative treatment (Figure 3). The goal of resection is a complete resection with negative microscopic margins, which commonly requires a partial hepatectomy to achieve this in the case of hilar cholangiocarcinoma and a pancreaticoduodenectomy in the case of a distal cholangiocarcinoma. Rarely, isolated mid bile duct resections can achieve an R0 resection of a small bile duct tumor.
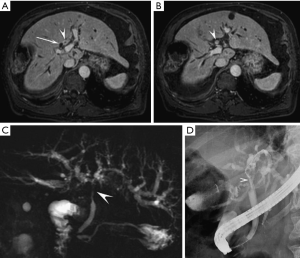
Hilar cholangiocarcinoma
Resectability of hilar cholangiocarcinoma is dependent on multiple factors (Tables 2,3). In addition to assessing the clinical status of the patient and their ability to undergo an extensive operation, preoperative imaging is required to assess the degree of bile duct and vascular involvement in order to determine stage (Table 4). Hepatic duct involvement with bilateral tumor extension to second order biliary radicals or encasement of the main portal vein are relative contraindications to curative resection. Tumor involving ipsilateral second order biliary radicals or ipsilateral portal vein branches may be amenable to resection while contralateral involvement would generally not be. Additionally, tumor that results in an ipsilateral lobar atrophy would not necessarily preclude resection, while contralateral atrophy likely would since this implies vascular involvement contralateral to the tumor. Series of resections for patients with extensive biliary and/or vascular involvement requiring vascular reconstruction have been reported but have not been universally accepted. Careful assessment is also required to rule out distant metastases to lymph nodes outside of the regional lymph node basin.

Full table
In patients that are deemed to have resectable disease and proceed to the operating room, up to one-half of patients will be found to have unresectable disease (48). Diagnostic laparoscopy for hilar cholangiocarcinoma is not routinely recommended. A recent review by Ruys et al. found that in 175 patients with hilar cholangiocarcinoma who underwent diagnostic laparoscopy, 24 were deemed unresectable for a yield of 14% (49). In addition, of the 76 patients that were unresectable, only 24 were identified by laparoscopy resulting in an accuracy of only 32%. Once the abdomen has been explored thoroughly and the absence of metastatic disease has been confirmed, tumor resection proceeds with en bloc hepatic resection. Very rarely is a segmental bile duct and biliary reconstruction sufficient and is generally discouraged due to the high likelihood of a positive margin (R1 resection). Routine en bloc hepatic resection with an extrahepatic bile duct resection results in R0 resection rates of 60–80% (50-54). This typically requires a major hepatic resection to include the caudate as part of either an extended right or left hepatectomy. Frozen section analysis should be performed to ensure an R0 bile duct resection margin. Patients with a secondary R0 resection margin after additional resection have been shown to have an equivalent survival (30.6 months) to those with a primary R0 resection (29.3 months), however, this is accompanied by an increase incidence of biliary fistula (44.4% vs. 17.5%, P=0.02) (45). It is important to note that the frozen section analysis of the bile duct margin is inaccurate in 9% of patients (55). Dysplasia at the margin (including high grade) has not been shown to be associated with worse outcomes (56).
Resection of the caudate lobe should be performed in almost all cases as a central biliary tumor at the confluence, by definition, involves the caudate ducts. Routine lymphadenectomy includes lymph nodes within the hepatoduodenal ligament extending from the level of the common hepatic artery and retroduodenal area up to the base of the liver. The extent of lymphadenectomy in patients with hilar cholangiocarcinoma continues to be an area of debate. Studies have shown that the presence of nodal metastasis beyond the hepatoduodenal ligament is associated with a dismal prognosis with 5-year overall survival rates reported to range 0–12.3% (57,58). As a result, an extended lymphadenectomy to include lymph nodes along the common hepatic artery or celiac axis is not recommended and any involvement of these nodal basins represents unresectable disease. For accurate staging, the estimated total lymph node count for hilar cholangiocarcinoma should be seven lymph nodes (54,59). Finally, biliary continuity is reestablished with a Roux-en-y hepaticojejunostomy.
For patients undergoing an R0 resection, the 5-year overall survival rates range between 20–40%, with a median overall survival of 30 to 40 months (48,52,60-62). Unfortunately, despite a potentially curative resection, recurrence is common. In a series of 76 patients, 52 patients (68%) developed a recurrence at a median time of 20 months. Recurrence location in this series was evenly split between local and distant sites (41% vs. 36%). Median overall survival following recurrence was 29 months. In a another study from Japan, Kobayaski et al. reported 3- and 4-year cumulative recurrence rates of 52% and 56% in a series of 79 patients (63). Distant recurrences were more common (81%) compared to locoregional recurrences (19%). Positive lymph nodes, moderate to poorly differentiated tumors, and stage T3/T4 were independent risk factors for recurrence.
Distal cholangiocarcinoma
Distal cholangiocarcinoma most commonly occurs within the pancreatic portion of the common bile duct and account for 30–40% of cholangiocarcinomas (40,60). Given their location, resection is almost exclusively limited to a pancreaticoduodenectomy. Very rarely are these tumors amenable to a segmental bile duct resection without compromising the oncologic integrity of the operation. In a series of 147 patients who underwent pancreaticoduodenectomy, 63% had evidence of nodal metastasis and 87% had evidence of pancreatic invasion (64). An R1 resection, moderate or poorly differentiated tumor, in addition to the presence of lymph node metastasis have been found to be independent adverse prognostic factors (59). Adequate lymphadenectomy for accurate staging should ideally include at least 11 lymph nodes (59). Peri-operative mortality for distal cholangiocarcinoma following pancreaticoduodenectomy has been reported to be approximately 3% (60). Five-year overall survival for distal cholangiocarcinoma, following R0 resection, ranges from 27–44% with median overall survival of 18 months (60,65). Due to the rarity of these tumors, as well as the surgical complexity, referral to a hepatobiliary specialized center for management is recommended.
Conclusions
Management of BTC tumors requires significant expertise in patient selection, interpretation of imaging, and technical surgery. As the incidence of these tumors increase, they continue to present a diagnostic and therapeutic challenge for clinicians. The common development of symptoms late in the disease process leads to the majority of patients presenting with locally unresectable or metastatic disease. In resectable, non-metastatic patients, complete resection remains the only potentially curative treatment. However, despite potentially curative surgery, recurrence rates remain high. Given the rarity of these tumors, further multi-institutional and international collaborative efforts are vital to continue to optimize management of these challenging cancers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ito H, Matros E, Brooks DC, et al. Treatment outcomes associated with surgery for gallbladder cancer: a 20-year experience. J Gastrointest Surg 2004;8:183-90. [Crossref] [PubMed]
- Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40. [Crossref] [PubMed]
- Konstantinidis IT, Deshpande V, Genevay M, et al. Trends in presentation and survival for gallbladder cancer during a period of more than 4 decades: a single-institution experience. Arch Surg 2009;144:441-7; discussion 447. [Crossref] [PubMed]
- Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan Kettering Cancer Centre (MSKCC). J Surg Oncol 2008;98:485-9. [Crossref] [PubMed]
- Hawkins WG, DeMatteo RP, Jarnagin WR, et al. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol 2004;11:310-5. [Crossref] [PubMed]
- Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg 2000;232:557-69. [Crossref] [PubMed]
- Shih SP, Schulick RD, Cameron JL, et al. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg 2007;245:893-901. [Crossref] [PubMed]
- Edge SB, Compton CC, Fritz AG, et al. AJCC cancer staging manual. New York: Springer, 2010.
- Ouchi K, Mikuni J, Kakugawa Y, et al. Laparoscopic cholecystectomy for gallbladder carcinoma: results of a Japanese survey of 498 patients. J Hepatobiliary Pancreat Surg 2002;9:256-60. [Crossref] [PubMed]
- Lee SE, Jang JY, Kim SW, et al. Surgical strategy for T1 gallbladder cancer: a nationwide multicenter survey in South Korea. Ann Surg Oncol 2014;21:3654-60. [Crossref] [PubMed]
- Sun CD, Zhang BY, Wu LQ, et al. Laparoscopic cholecystectomy for treatment of unexpected early-stage gallbladder cancer. J Surg Oncol 2005;91:253-7. [Crossref] [PubMed]
- Toyonaga T, Chijiiwa K, Nakano K, et al. Completion radical surgery after cholecystectomy for accidentally undiagnosed gallbladder carcinoma. World J Surg 2003;27:266-71. [Crossref] [PubMed]
- Wakai T, Shirai Y, Yokoyama N, et al. Early gallbladder carcinoma does not warrant radical resection. Br J Surg 2001;88:675-8. [Crossref] [PubMed]
- Abramson MA, Pandharipande P, Ruan D, et al. Radical resection for T1b gallbladder cancer: a decision analysis. HPB (Oxford) 2009;11:656-63. [Crossref] [PubMed]
- Shukla PJ, Barreto G, Kakade A, et al. Revision surgery for incidental gallbladder cancer: factors influencing operability and further evidence for T1b tumours. HPB (Oxford) 2008;10:43-7. [Crossref] [PubMed]
- You DD, Lee HG, Paik KY, et al. What is an adequate extent of resection for T1 gallbladder cancers? Ann Surg 2008;247:835-8. [Crossref] [PubMed]
- Wagholikar GD, Behari A, Krishnani N, et al. Early gallbladder cancer. J Am Coll Surg 2002;194:137-41. [Crossref] [PubMed]
- Are C. Common controversies in the management of gallbladder cancer. J Natl Compr Canc Netw 2014;12:833-5. [PubMed]
- Benson AB 3rd, Abrams TA, Ben-Josef E, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw 2009;7:350-91. [PubMed]
- Aloia TA, Járufe N, Javle M, et al. Gallbladder cancer: expert consensus statement. HPB (Oxford) 2015;17:681-90. [Crossref] [PubMed]
- Yokomizo H, Yamane T, Hirata T, et al. Surgical treatment of pT2 gallbladder carcinoma: a reevaluation of the therapeutic effect of hepatectomy and extrahepatic bile duct resection based on the long-term outcome. Ann Surg Oncol 2007;14:1366-73. [Crossref] [PubMed]
- D'Angelica M, Dalal KM, DeMatteo RP, et al. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol 2009;16:806-16. [Crossref] [PubMed]
- Choi SB, Han HJ, Kim CY, et al. Surgical outcomes and prognostic factors for T2 gallbladder cancer following surgical resection. J Gastrointest Surg 2010;14:668-78. [Crossref] [PubMed]
- Fong Y, Heffernan N, Blumgart LH. Gallbladder carcinoma discovered during laparoscopic cholecystectomy: aggressive reresection is beneficial. Cancer 1998;83:423-7. [Crossref] [PubMed]
- Foster JM, Hoshi H, Gibbs JF, et al. Gallbladder cancer: Defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol 2007;14:833-40. [Crossref] [PubMed]
- Chijiiwa K, Nakano K, Ueda J, et al. Surgical treatment of patients with T2 gallbladder carcinoma invading the subserosal layer. J Am Coll Surg 2001;192:600-7. [Crossref] [PubMed]
- Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg 1994;219:275-80. [Crossref] [PubMed]
- Wakabayashi H, Ishimura K, Hashimoto N, et al. Analysis of prognostic factors after surgery for stage III and IV gallbladder cancer. Eur J Surg Oncol 2004;30:842-6. [Crossref] [PubMed]
- Kang CM, Choi GH, Park SH, et al. Laparoscopic cholecystectomy only could be an appropriate treatment for selected clinical R0 gallbladder carcinoma. Surg Endosc 2007;21:1582-7. [Crossref] [PubMed]
- Shimizu Y, Ohtsuka M, Ito H, et al. Should the extrahepatic bile duct be resected for locally advanced gallbladder cancer? Surgery 2004;136:1012-7; discussion 1018. [Crossref] [PubMed]
- Kohya N, Miyazaki K. Hepatectomy of segment 4a and 5 combined with extra-hepatic bile duct resection for T2 and T3 gallbladder carcinoma. J Surg Oncol 2008;97:498-502. [Crossref] [PubMed]
- Ito H, Ito K, D'Angelica M, et al. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg 2011;254:320-5. [Crossref] [PubMed]
- Downing SR, Cadogan KA, Ortega G, et al. Early-stage gallbladder cancer in the Surveillance, Epidemiology, and End Results database: effect of extended surgical resection. Arch Surg 2011;146:734-8. [Crossref] [PubMed]
- Mayo SC, Shore AD, Nathan H, et al. National trends in the management and survival of surgically managed gallbladder adenocarcinoma over 15 years: a population-based analysis. J Gastrointest Surg 2010;14:1578-91. [Crossref] [PubMed]
- Weber SM, DeMatteo RP, Fong Y, et al. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg 2002;235:392-9. [Crossref] [PubMed]
- Butte JM, Gonen M, Allen PJ, et al. The role of laparoscopic staging in patients with incidental gallbladder cancer. HPB (Oxford) 2011;13:463-72. [Crossref] [PubMed]
- Maker AV, Butte JM, Oxenberg J, et al. Is port site resection necessary in the surgical management of gallbladder cancer? Ann Surg Oncol 2012;19:409-17. [Crossref] [PubMed]
- Shoup M, Fong Y. Surgical indications and extent of resection in gallbladder cancer. Surg Oncol Clin N Am 2002;11:985-94. [Crossref] [PubMed]
- Povoski SP, Ouellette JR, Chang WW, et al. Axillary lymph node metastasis following resection of abdominal wall laparoscopic port site recurrence of gallbladder cancer. J Hepatobiliary Pancreat Surg 2004;11:197-202. [Crossref] [PubMed]
- Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996;224:463-73; discussion 473-5. [Crossref] [PubMed]
- Lieser MJ, Barry MK, Rowland C, et al. Surgical management of intrahepatic cholangiocarcinoma: a 31-year experience. J Hepatobiliary Pancreat Surg 1998;5:41-7. [Crossref] [PubMed]
- de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-5. [Crossref] [PubMed]
- Spolverato G, Vitale A, Cucchetti A, et al. Can hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma? Cancer 2015;121:3998-4006. [Crossref] [PubMed]
- Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84-96. [Crossref] [PubMed]
- Ribero D, Amisano M, Lo Tesoriere R, et al. Additional resection of an intraoperative margin-positive proximal bile duct improves survival in patients with hilar cholangiocarcinoma. Ann Surg 2011;254:776-81; discussion 781-3. [Crossref] [PubMed]
- Morine Y, Shimada M, Utsunomiya T, et al. Clinical impact of lymph node dissection in surgery for peripheral-type intrahepatic cholangiocarcinoma. Surg Today 2012;42:147-51. [Crossref] [PubMed]
- Amini N, Ejaz A, Spolverato G, et al. Management of lymph nodes during resection of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: a systematic review. J Gastrointest Surg 2014;18:2136-48. [Crossref] [PubMed]
- Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001;234:507-17; discussion 517-9. [Crossref] [PubMed]
- Ruys AT, Busch OR, Gouma DJ, et al. Staging laparoscopy for hilar cholangiocarcinoma: is it still worthwhile? Ann Surg Oncol 2011;18:2647-53. [Crossref] [PubMed]
- Miyazaki M, Kimura F, Shimizu H, et al. One hundred seven consecutive surgical resections for hilar cholangiocarcinoma of Bismuth types II, III, IV between 2001 and 2008. J Hepatobiliary Pancreat Sci 2010;17:470-5. [Crossref] [PubMed]
- Lee SG, Song GW, Hwang S, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci 2010;17:476-89. [Crossref] [PubMed]
- Nuzzo G, Giuliante F, Ardito F, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg 2012;147:26-34. [Crossref] [PubMed]
- Regimbeau JM, Fuks D, Le Treut YP, et al. Surgery for hilar cholangiocarcinoma: a multi-institutional update on practice and outcome by the AFC-HC study group. J Gastrointest Surg 2011;15:480-8. [Crossref] [PubMed]
- Rocha FG, Matsuo K, Blumgart LH, et al. Hilar cholangiocarcinoma: the Memorial Sloan Kettering Cancer Center experience. J Hepatobiliary Pancreat Sci 2010;17:490-6. [Crossref] [PubMed]
- Endo I, House MG, Klimstra DS, et al. Clinical significance of intraoperative bile duct margin assessment for hilar cholangiocarcinoma. Ann Surg Oncol 2008;15:2104-12. [Crossref] [PubMed]
- Han IW, Jang JY, Lee KB, et al. Clinicopathological analysis and prognosis of extrahepatic bile duct cancer with a microscopic positive ductal margin. HPB (Oxford) 2014;16:575-81. [Crossref] [PubMed]
- Kitagawa Y, Nagino M, Kamiya J, et al. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg 2001;233:385-92. [Crossref] [PubMed]
- Kosuge T, Yamamoto J, Shimada K, et al. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg 1999;230:663-71. [Crossref] [PubMed]
- Ito K, Ito H, Allen PJ, et al. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg 2010;251:675-81. [Crossref] [PubMed]
- DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007;245:755-62. [Crossref] [PubMed]
- Cho MS, Kim SH, Park SW, et al. Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J Gastrointest Surg 2012;16:1672-9. [Crossref] [PubMed]
- Silva MA, Tekin K, Aytekin F, et al. Surgery for hilar cholangiocarcinoma; a 10 year experience of a tertiary referral centre in the UK. Eur J Surg Oncol 2005;31:533-9. [Crossref] [PubMed]
- Kobayashi A, Miwa S, Nakata T, et al. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br J Surg 2010;97:56-64. [Crossref] [PubMed]
- Hong SM, Pawlik TM, Cho H, et al. Depth of tumor invasion better predicts prognosis than the current American Joint Committee on Cancer T classification for distal bile duct carcinoma. Surgery 2009;146:250-7. [Crossref] [PubMed]
- Yoshida T, Matsumoto T, Sasaki A, et al. Prognostic factors after pancreatoduodenectomy with extended lymphadenectomy for distal bile duct cancer. Arch Surg 2002;137:69-73. [Crossref] [PubMed]


