Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency
Introduction
The bile duct system follows the passage of the bile from its production by the liver cells through the intra- and extra-hepatic bile ducts to the duodenum. Along this passageway, the gallbladder serves as a storage organ, storing up to 50% of the bile and releasing it when food is ingested.
Cancers that arise from the various segments of the biliary system exhibit varied clinico-pathological characteristics. Thus, they are traditionally separated into categories based on their specific location in the system: gallbladder carcinoma, intra-hepatic cholangiocarcinoma, hilar or perihilar cholangiocarcinoma, carcinoma of the distal common bile duct, and ampullary carcinoma (1).
Tumorigenesis of the biliary epithelium in general follows the dysplasia to carcinoma sequence. The initiation and progression of this process is known to be heavily influenced by various risk factors including: (I) chronic inflammation related to insults such as biliary lithiasis, hepatobiliary flukes, primary sclerosing cholangitis and viral hepatitis; (II) developmental anomalies such as abnormal junction of the pancreatic and bile ducts, and choledochal cysts; and (III) other environmental or metabolic factors such as smoking and diabetes. Consequently, there exists a geographic difference in cancer incidence with the highest incidence occurring in East and South Asia and parts of South America, as well as a trend towards an increasingly higher global incidence (2,3).
At the molecular level, a number of mutational targets have been implicated in these tumors including KRAS, NRAS, GNAS, EGFR, TP53, DCKN2A, BRAF, and CTNNB1 (4,5). Recent genomic profiling studies have shed further light into the genetic makeup of biliary cancers (5-8). It is becoming increasingly clearer that genomic alterations differ in tumors of the different segments of the biliary system, and data are emerging revealing alterations such as increased levels of EGFR and ERBB2 signaling, that are clinically actionable, in biliary tumors.
DNA mismatch repair (MMR) deficiency leading to microsatellite instability (MSI) has been recognized as a distinct tumorigenesis pathway (9,10). Lynch syndrome, defined by deleterious germline mutation in one of the four major MMR genes (MLH1, MSH2, MSH6 and PMS2) or the EPCAM gene, represents the hereditary prototype of MMR deficiency leading to tumor formation. MLH1 promoter methylation is by far the most prevalent mechanism causing sporadic MMR deficient cancers. Although MSI most commonly occurs in colorectal and endometrial cancers, a wide variety of other tumor types, including biliary cancers, have been shown to exhibit MSI as well (11-13). Understanding the role of MMR deficiency in such non-colorectal tumors may not only benefit the detection and management of Lynch syndrome, it bears implication in the management of sporadic cancer patients as well.
In this article, we provide a summary of the pathology of the biliary carcinomas, and using this summary as a basis, we further examine the current status on our understanding of the role of DNA MMR deficiency in biliary carcinomas.
The pathology of biliary carcinomas
Gallbladder carcinoma
Gallbladder carcinomas tend to have a diffuse and scirrhous pattern of growth, and are less likely to form a distinct mass. A luminal polypoid component may be present, and when it occurs, this component often represents an intracystic papillary neoplasm. Upon sectioning, the infiltrative tumor is often firm and gritty. Gallstones are often present as gallbladder carcinomas are often associated with gallstones. In cases of porcelain gallbladder, the wall of the gallbladder is typically rigid and calcified.
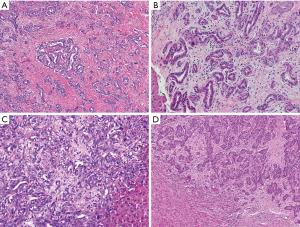
Histologically, gallbladder carcinomas may evolve from different forms of precursor lesions and can assume various phenotypical subtypes, as outlined by the 4th edition of the WHO classification of tumors of the digestive system (Table 1) (14). The microscopic appearance varies depending on the histological subtype. The majority show features typical of pancreatobiliary-type adenocarcinoma. Tumors may invade the wall in a “non-continuous” fashion and appear as haphazardly distributed foci (Figure 1A), or grow in continuity with mucosal dysplasia (Figure 1B). Characteristically, the tumor cells form glands or grow in cords and nests, with or without conspicuous mucin (Figure 1C). As with all biliary carcinomas, tumors can be poorly differentiated, and not infrequently, they have “squamoid” features (Figure 1D), and overt adenosquamous carcinoma may happen.

Full table

Gallbladder carcinoma may spread loco-regionally to the liver or adjacent structures by direct extension or to the regional lymph nodes through the lymphatic drainage (15). The 7th edition AJCC staging manual (16) defines N1 disease by metastases to nodes along the cystic duct, common bile duct, hepatic artery and portal vein, and N2 disease by metastases to periaortic, pericaval, superior mesenteric artery, and/or celiac artery lymph nodes. Distant metastases of the gallbladder carcinoma occur via hematogenous spread, and typically target sites such as the peritoneum, liver, and occasionally to the lungs and pleura.
Intrahepatic cholangiocarcinoma
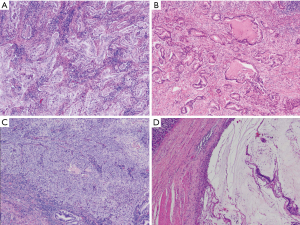
These tumors typically occur as well circumscribed masses. Less commonly, they may assume a periductal infiltrating pattern or a mixed pattern of mass forming and periductal infiltrating type (17). Histologically, the tumor cells show resemblance to interlobular bile ducts with cuboidal cells and typically with no mucin production (Figure 2A,B), and the tumors characteristically have ample amount of desmoplastic stroma. Histological variations occur not infrequently and tumors may be spindly and sarcomatoid (Figure 2C) or with squamous differentiation (Figure 2D). A unique challenge in the diagnosis of intrahepatic cholangiocarcinoma relates to the fact that many extra-hepatic adenocarcinomas (such as adenocarcinomas of the upper gastrointestinal tract, breast, and lung) that can show similar morphological patterns can metastasize to the liver, rendering determination of site of origin difficult or, in some cases, impossible, on pure histologic grounds. Notably, the recent development of branched DNA-enhanced albumin RNA in situ hybridization technique has afforded the promise of high accuracy diagnosis of hepatic primary with a reported sensitivity of 99% (18); intrahepatic cholangiocarcinomas show positive signal whereas metastatic tumors do not.
Biliary intraepithelial neoplasia (BilIN) is well accepted to be a pre-malignant lesion for biliary adenocarcinoma. Within the liver, BilIN is more commonly detected in the setting of primary sclerosing cholangitis-associated carcinoma. The intriguing question of whether some intrahepatic cholangiocarcinomas develop from a pre-existing bile duct adenoma remains to be answered.
Mucinous cystic neoplasm (MCN) of the liver represents another unique form of pre-malignant neoplasia. It is characterized by well encapsulated multiloculated cystic lesion with ovarian-type stroma. The lining epithelium may be columnar, cuboidal, or flattened, and is mucus-secreting. This lesion can give rise to invasive adenocarcinoma that typically is of ductal type with tubulopapillary or tubular growth patterns. The associated MCN distinguishes these carcinomas from conventional intrahepatic cholangiocarcinoma.
The pattern of spread of intrahepatic cholangiocarcinomas differs according to tumor location (15). Tumors occurring in the left lateral bi-segments (segment 2–3) of the liver may preferentially drain to lymph nodes along the lesser curvature of the stomach and subsequently to the celiac nodal basin, whereas tumors in the right liver (segment 5–8) may preferentially drain to hilar lymph nodes and subsequently to caval and periaortic lymph nodes. Involvement of celiac and/or periaortic and caval lymph nodes are considered distant metastasis according to the 7th edition AJCC staging manual (16). Hematogenous spread of the tumor may occur within the organ or causing tumor spread to sites such as the peritoneum, lungs, pleura, bone, adrenals, kidneys spleen and pancreas. Notably, intrahepatic cholangiocarcinoma metastasizing to other intrahepatic locations is classified in the T category (as multiple tumors, similar to that for hepatocellular carcinoma), not M, by the AJCC (16).
Carcinoma of the extra-hepatic bile ducts
Carcinomas of the extrahepatic bile ducts, including the hilar hepatic ducts, cystic duct, and common bile duct, can be classified into similar histologic types as carcinomas of the gallbladder. In fact, the WHO (14) advocates one histologic classification system for both the gallbladder and the extrahepatic bile ducts (Table 1). Features that appear more unique to the bile ducts as compared to the gallbladder are often attributable to their unique anatomic location. Grossly, because of the relatively thin wall of the bile ducts, even small (<1.0 cm) carcinomas invade deeply into, or through, the wall and into adjacent soft tissue, liver, or pancreas. Very commonly, carcinomas of the bile ducts merge imperceptible with adjacent areas of inflammation and fibrosis, forming poorly-defined gross lesions, and necessitating frozen section diagnosis for margin status at the time of surgery.
Carcinoma of the extrahepatic bile ducts may spread via lymphatic drainage to hilar and pericholedochal nodes in the hepatoduodenal ligament, and subsequently to periaortic, pericaval, superior mesenteric artery, and/or celiac artery lymph nodes. Similar to gallbladder carcinomas and intrahepatic cholangiocarcinomas, carcinomas of the extrahepatic bile ducts may spread to the peritoneum and distally to the lungs and pleura, although the frequency of distant metastasis seems relatively low.
Ampullary carcinoma
This tumor type represents carcinomas of the terminal portion of the bile duct. Tumors in this region, however, may also derive from duodenal epithelium or pancreatic ductal epithelium. Consequently, a variety of histological patterns can occur, including pancreatobiliary type (Figure 3A), intestinal type (Figure 3B), mixed type, and other variants including poorly differentiated (Figure 3C) and mucinous type (Figure 3D) (Table 1). Histologic classification can be challenging in cases with mixed or varied histological patterns, and immunohistochemical markers may be helpful in such situations (19).
Pre-malignant lesions in the ampullary region include BilIN, and ampullary or duodenal adenoma. The natural history of sporadic adenomas in this region (outside the setting of familial adenomatous polyposis) remains to be defined. The detection of a mass forming ampullary adenoma often results in surgical removal that not infrequently involves pancreatoduodenectomy. A substantial subset of ampullary adenomas diagnosed on biopsy harbor invasive carcinoma upon resection (20).
Carcinomas of the ampulla may spread through the sphincter of Oddi to the duodenum or the pancreas. Nodal metastasis occurs most commonly in the peripancreatic lymph nodes. Distant spread often involves liver, peritoneum, lungs and pleura.
DNA MMR deficiency in biliary carcinomas
Literature review
Pertinent and comparable studies on MMR deficiency in biliary carcinomas are summarized in Table 2.
Full table
MMR deficiency in gallbladder carcinoma
MMR deficiency, mostly measured by PCR based MSI testing, with a few studies utilizing MMR protein immunohistochemistry (IHC), has been detected in some gallbladder carcinomas (21-30,46). The reported frequencies of MSI-H varied from 0% to 42%, and averaged 5% overall (after weighing for sample size) (Table 2). Most studies were performed on populations with high prevalence of gallbladder carcinoma; frequency studies in the North American population are scarce.
Several characteristics about MMR deficiency in gallbladder tumors have emerged from the literature. First, its occurrence has been found to be more frequent in gallbladder carcinomas from patients with abnormal junction of the pancreatic and bile ducts (46,47). Second, it is present not only in carcinomas, but in some cases, in severe chronic cholecystitis (25) and dysplastic lesions (25,27) as well, suggesting that the involvement of MMR deficiency in tumor development may be related to chronic inflammatory injury and occurs early in the tumorigenic process.
In a recent study (30), loss of MSH2 and MSH6 by IHC was found to be relatively common in MMR-deficient gallbladder carcinomas, however, this was not associated with clinical traits of Lynch syndrome (personal and family cancer history), suggesting MMR deficiency in gallbladder carcinomas may not represent a manifestation of Lynch syndrome. Corroborating with this finding, MSI gallbladder cancers lacked strong LINE-1 RNA expression indicating high methylation status, thus suggesting that methylation is likely a major mechanism leading to MMR deficiency.
Thus far, no particular association has been established between MMR deficiency and tumor grade or tumor morphology (mucin production or tumor infiltrating lymphocytes) in gallbladder carcinomas. Clinically, a trend towards a better prognosis in MSI-H cancers has been suggested (27); however, this remains to be confirmed.
MMR deficiency in intrahepatic cholangiocarcinoma
Most studies came from analyses of patients in Southeast Asia, particularly Thailand where liver cholangiocarcinoma represents one of the most common cancers and is believed to be associated with liver fluke (opisthorchis viverrini) infection (48).
Results of MSI are somewhat inconsistent attributable to different studies using different microsatellite markers and different definitions (31-35). Two studies reported rates of 13-20% of MSI; however, neither study detected instability in mononucleotide repeats. On the other hand, a study by Liengswangwong et al. (34) that used the NCI criteria (49), reported that none of their 37 intra-hepatic cholangiocarcinomas exhibited MSI-H. This group of patients was further evaluated by MMR IHC in a subsequent study (35). In this IHC study (35), 5 of 29 tumors showed either MLH1 or MSH2 loss, for a total rate of MMR deficiency of 17%. Of note, in this study, one case showed concurrent loss of MLH1 and MSH2, a pattern almost non-exist in colorectal MSI cancers (50). Overall, the frequency of MSI-H in intrahepatic cholangiocarcinomas based on studies with relatively comparable data was 10%.
The low number of cases studied in the available studies makes it difficult to further assess whether the frequency of MMR deficiency differ among patients with different etiologies. Also not systematically addressed in the existing studies is the question whether and how MMR deficiency impact on tumor pathology and clinical behavior.
MMR deficiency in extra-hepatic bile duct carcinomas
Reports on MMR deficiency in extra-hepatic bile duct carcinomas are scarce. A study by Suto et al. (36) evaluated MSI in 38 tumors using 7 microsatellite markers, and found 5 (13%) showing instability at 1 or 2 loci, none involving mononucleotide markers, and none fulfilling the MSI-H criterion that requires 40% or more of the tested loci being instable. Another study found 2 of 28 (7%) tumors showing MSI-H defined as instability at 40% of the 6 tested markers, both tumors showed mononucleotide instability. Similarly, Kim et al. (38) used the five NCI panel markers, and found MSI-H in 1 of 18 carcinomas (6%). Overall, the reported frequency of MSI-H in carcinomas of the large bile ducts is estimated to be 5% (Table 2).
Notably, a recent case report (39) described the occurrence of a carcinoma of the hilar hepatic duct in a 73-year-old patient carrying a germline mutation in MLH1, c.209_211delAAG. This patient had a personal and family cancer history fulfilling Amsterdam Criteria II. The hepatic duct carcinoma was found to have lost MLH1 protein by IHC and PCR MSI testing revealed instability in 4 of the 5 NCI panel markers. Very significantly, the bile duct carcinoma was detected at an early stage (pT1) and the early diagnosis was enabled by tests prompted by periodic surveillance blood examination showing abnormal values of hepatobiliary enzymes. Thus, this case not only documents the occurrence of bile duct carcinoma manifesting the underlying MMR gene abnormality in Lynch syndrome, it also illustrates the importance of continuous surveillance for extracolonic tumors, including bile duct cancers, in patients with Lynch syndrome. However, as yet, cost-effective strategies for such tumor surveillance remain to be determined.
Further systematic analyses on the association of MMR deficiency in bile duct carcinomas with tumor pathology or clinical behavior are essentially lacking at the current time.
MMR deficiency in ampullary carcinomas
Among all biliary carcinomas, ampullary tumors are most studied with regard to MMR deficiency (23,36,38,40,42-45,51). While the reported frequencies of MSI or MMR IHC abnormality differed in different studies, the fact MMR deficiency occurs in some ampullary carcinomas is well established. Overall, the reported frequency of MSI-H in ampullary carcinoma is 10% (Table 2). Using IHC with all four MMR proteins, we found MMR protein loss in 6% of ampullary carcinomas (45). Our study and that of others have allowed the following conclusions about MMR deficiency in ampullary carcinomas.
First, MMR deficient ampullary tumors can occur in Lynch syndrome. Although data are still limited, there seems to be a tendency towards ampullary tumors occurring in individuals with germline mutation in MSH6, and tumors may occur at an old age. Notably, however, at the current time, there is no evidence yet to suggest that any ampullary carcinoma should trigger MMR immunohistochemical/MSI testing or genetic testing, but it appears reasonable that, in families at high risk for Lynch syndrome, when more conventional tumor types are not available, ampullary carcinoma be used as a tumor sample for detection of MMR protein or function. In terms of surveillance for ampullary carcinomas in patients with Lynch syndrome, standard recommendations are lacking and similar to the case of bile duct carcinoma, cost-effective detection modalities specifically targeting ampullary tumors are still to be determined.
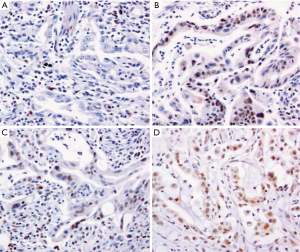
Second, MMR deficient ampullary tumors bear certain pathological features. It has been observed not only in carcinomas, but also in adenomas, suggesting it to be an early event in the tumorigenesis process (44). At the carcinoma stage, MMR deficiency has been associated with both the intestinal (43,44) and the pancreatobiliary (45) subtypes. Furthermore, there is also a trend for MMR deficient ampullary carcinomas to show morphological patterns similar to that seen in MMR deficient colorectal cancers, particularly increased tumor infiltrating lymphocytes and poor differentiation with “medullary” like histology (Figures 4,5) (44,45).
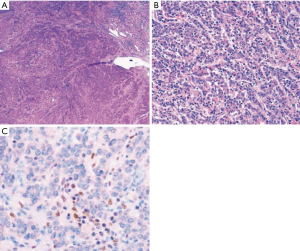
Clinically, MSI-H ampullary carcinomas have been shown to have a significantly longer overall survival than MSI-L or MSS tumors (44), although such prognostic impact remains to be further confirmed.
Summary
Overall, the literature data on MMR deficiency in biliary cancers are limited. Factors affecting the accumulation of comparable data include small sample size, varied study populations, different microsatellite markers as well as different definitions for MSI, different antibody panels and interpretational variations for MMR IHC, and lack of detailed correlation with tumor pathology and biological behavior.
Nonetheless, it can be gleaned from the above review that (I) MMR deficiency indeed occurs in biliary carcinomas, albeit at low frequencies; (II) while Lynch syndrome families are known to carry an increased risk of developing biliary tumor [estimated risk to age 70, 1.4–4.1% (13,52,53)], this risk seems site-specific: association with Lynch syndrome as yet unproven in gallbladder carcinomas where MMR deficiency seems to be primarily a sporadic occurrence likely resulting from abnormal methylation, but proven in bile duct and ampullary carcinomas where tumor onset may be late; and (III) MMR deficient biliary cancers may bear other unique pathological and clinical characteristics, warranting further investigation.
Conclusions and future perspectives
Biliary carcinomas represent a heterogeneous group of tumors resulting from a complex interplay among different causative factors and different microenvironment throughout the biliary system. Consequently, significant heterogeneity exists in tumor pathology and the underlying molecular alterations. It seems certain that DNA MMR deficiency plays a role in some biliary tumors. This role, however, may differ from that seen in other conventional Lynch syndrome associated cancer types, and may vary even within the family of biliary tumors.
In the current era of next generation sequencing, it can be anticipated that large scale genomic analysis will allow a more integrated view of the molecular alterations of the various biliary tumors and allow the detection of clinically actionable gene targets. Indeed, a recent study (8) utilizing whole exome sequencing on a series of biliary carcinomas detected an overall 5.9% (14/239) “hypermutated” tumors, and found that some such hypermutated tumors harbored inactivating (nonsense, frameshift or spice-site) mutations in mismatch-repair complex components. More importantly, the analysis detected that cases with the poorest prognosis had significant enrichment of hypermutated tumors and a characteristic elevation in the expression of immune checkpoint molecules, suggesting immune-modulating therapies might be potentially beneficial for these patients (8,54).
Given the significant tumor heterogeneity, it is imperative that future efforts on achieving effective detection of clinically actionable molecular targets be site and type specific, and utilizes a multidisciplinary approach integrating genomic discoveries with not only functional studies but also studies of tumor pathology and the tumor’s clinical and biological behavior.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Albores-Saavedra J, Henson DE, Klimstra DS. Tumors of the Gallbladder, Extrahepatic Bile Ducts, and Ampulla of Vater. Atlas of Tumor Pathology; 3rd series, fascicle 27. Washington, DC: Armed Forces Institute of Pathology, 2000.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013;145:1215-29. [Crossref] [PubMed]
- Marsh Rde W, Alonzo M, Bajaj S, et al. Comprehensive review of the diagnosis and treatment of biliary tract cancer 2012. Part I: diagnosis-clinical staging and pathology. J Surg Oncol 2012;106:332-8. [Crossref] [PubMed]
- Jang S, Chun SM, Hong SM, et al. High throughput molecular profiling reveals differential mutation patterns in intrahepatic cholangiocarcinomas arising in chronic advanced liver diseases. Mod Pathol 2014;27:731-9. [Crossref] [PubMed]
- Andersen JB, Spee B, Blechacz BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology 2012;142:1021-1031.e15. [Crossref] [PubMed]
- Sia D, Hoshida Y, Villanueva A, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology 2013;144:829-40. [Crossref] [PubMed]
- Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015;47:1003-10. [Crossref] [PubMed]
- Shia J. Evolving approach and clinical significance of detecting DNA mismatch repair deficiency in colorectal carcinoma. Semin Diagn Pathol 2015;32:352-61. [Crossref] [PubMed]
- Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010;138:2073-2087.e3. [Crossref] [PubMed]
- Karamurzin Y, Zeng Z, Stadler ZK, et al. Unusual DNA mismatch repair-deficient tumors in Lynch syndrome: a report of new cases and review of the literature. Hum Pathol 2012;43:1677-87. [Crossref] [PubMed]
- Vasen HF. What is hereditary nonpolyposis colorectal cancer (HNPCC). Anticancer Res 1994;14:1613-5. [PubMed]
- Watson P, Vasen HF, Mecklin JP, et al. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer 2008;123:444-9. [Crossref] [PubMed]
- Bosman, FT, Carneiro F, Hruban RH, et al. editors. WHO Classification of Tumours of the Digestive System. Lyon: IARC, 2010.
- Ito K, Ito H, Allen PJ, et al. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg 2010;251:675-81. [Crossref] [PubMed]
- Edge S, Byrd DR, Carducci MA, et al. editors. AJCC cancer staging manual. 7th edition. New York: Springer, 2009.
- Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg 2003;10:288-91. [Crossref] [PubMed]
- Ferrone CR, Ting DT, Shahid M, et al. The Ability to Diagnose Intrahepatic Cholangiocarcinoma Definitively Using Novel Branched DNA-Enhanced Albumin RNA In Situ Hybridization Technology. Ann Surg Oncol 2016;23:290-6. [Crossref] [PubMed]
- Ang DC, Shia J, Tang LH, et al. The utility of immunohistochemistry in subtyping adenocarcinoma of the ampulla of vater. Am J Surg Pathol 2014;38:1371-9. [Crossref] [PubMed]
- Roggin KK, Yeh JJ, Ferrone CR, et al. Limitations of ampullectomy in the treatment of nonfamilial ampullary neoplasms. Ann Surg Oncol 2005;12:971-80. [Crossref] [PubMed]
- Yoshida T, Sugai T, Habano W, et al. Microsatellite instability in gallbladder carcinoma: two independent genetic pathways of gallbladder carcinogenesis. J Gastroenterol 2000;35:768-74. [Crossref] [PubMed]
- Kim YT, Kim J, Jang YH, et al. Genetic alterations in gallbladder adenoma, dysplasia and carcinoma. Cancer Lett 2001;169:59-68. [Crossref] [PubMed]
- Rashid A, Ueki T, Gao YT, et al. K-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: a population-based study in China. Clin Cancer Res 2002;8:3156-63. [PubMed]
- Sessa F, Furlan D, Genasetti A, et al. Microsatellite instability and p53 expression in gallbladder carcinomas. Diagn Mol Pathol 2003;12:96-102. [Crossref] [PubMed]
- Yanagisawa N, Mikami T, Yamashita K, et al. Microsatellite instability in chronic cholecystitis is indicative of an early stage in gallbladder carcinogenesis. Am J Clin Pathol 2003;120:413-7. [Crossref] [PubMed]
- Saetta AA, Papanastasiou P, Michalopoulos NV, et al. Mutational analysis of BRAF in gallbladder carcinomas in association with K-ras and p53 mutations and microsatellite instability. Virchows Arch 2004;445:179-82. [Crossref] [PubMed]
- Roa JC, Roa I, Correa P, Vo Q, et al. Microsatellite instability in preneoplastic and neoplastic lesions of the gallbladder. J Gastroenterol 2005;40:79-86. [Crossref] [PubMed]
- Saetta AA, Gigelou F, Papanastasiou PI, et al. High-level microsatellite instability is not involved in gallbladder carcinogenesis. Exp Mol Pathol 2006;80:67-71. [Crossref] [PubMed]
- Nagahashi M, Ajioka Y, Lang I, et al. Genetic changes of p53, K-ras, and microsatellite instability in gallbladder carcinoma in high-incidence areas of Japan and Hungary. World J Gastroenterol 2008;14:70-5. [Crossref] [PubMed]
- Moy AP, Shahid M, Ferrone CR, et al. Microsatellite instability in gallbladder carcinoma. Virchows Arch 2015;466:393-402. [Crossref] [PubMed]
- Momoi H, Itoh T, Nozaki Y, et al. Microsatellite instability and alternative genetic pathway in intrahepatic cholangiocarcinoma. J Hepatol 2001;35:235-44. [Crossref] [PubMed]
- Limpaiboon T, Krissadarak K, Sripa B, et al. Microsatellite alterations in liver fluke related cholangiocarcinoma are associated with poor prognosis. Cancer Lett 2002;181:215-22. [Crossref] [PubMed]
- Liu D, Momoi H, Li L, et al. Microsatellite instability in thorotrast-induced human intrahepatic cholangiocarcinoma. Int J Cancer 2002;102:366-71. [Crossref] [PubMed]
- Liengswangwong U, Nitta T, Kashiwagi H, et al. Infrequent microsatellite instability in liver fluke infection-associated intrahepatic cholangiocarcinomas from Thailand. Int J Cancer 2003;107:375-80. [Crossref] [PubMed]
- Liengswangwong U, Karalak A, Morishita Y, et al. Immunohistochemical expression of mismatch repair genes: a screening tool for predicting mutator phenotype in liver fluke infection-associated intrahepatic cholangiocarcinoma. World J Gastroenterol 2006;12:3740-5. [Crossref] [PubMed]
- Suto T, Habano W, Sugai T, et al. Infrequent microsatellite instability in biliary tract cancer. J Surg Oncol 2001;76:121-6. [Crossref] [PubMed]
- Abraham SC, Lee JH, Boitnott JK, et al. Microsatellite instability in intraductal papillary neoplasms of the biliary tract. Mod Pathol 2002;15:1309-17. [Crossref] [PubMed]
- Kim SG, Chan AO, Wu TT, et al. Epigenetic and genetic alterations in duodenal carcinomas are distinct from biliary and ampullary carcinomas. Gastroenterology 2003;124:1300-10. [Crossref] [PubMed]
- Shigeyasu K, Tanakaya K, Nagasaka T, et al. Early detection of metachronous bile duct cancer in Lynch syndrome: report of a case. Surg Today 2014;44:1975-81. [Crossref] [PubMed]
- Achille A, Biasi MO, Zamboni G, et al. Cancers of the papilla of vater: mutator phenotype is associated with good prognosis. Clin Cancer Res 1997;3:1841-7. [PubMed]
- Imai Y, Tsurutani N, Oda H, et al. Genetic instability and mutation of the TGF-beta-receptor-II gene in ampullary carcinomas. Int J Cancer 1998;76:407-11. [Crossref] [PubMed]
- Park S, Kim SW, Kim SH, et al. Lack of microsatellite instability in neoplasms of ampulla of Vater. Pathol Int 2003;53:667-70. [Crossref] [PubMed]
- Sessa F, Furlan D, Zampatti C, et al. Prognostic factors for ampullary adenocarcinomas: tumor stage, tumor histology, tumor location, immunohistochemistry and microsatellite instability. Virchows Arch 2007;451:649-57. [Crossref] [PubMed]
- Ruemmele P, Dietmaier W, Terracciano L, et al. Histopathologic features and microsatellite instability of cancers of the papilla of vater and their precursor lesions. Am J Surg Pathol 2009;33:691-704. [Crossref] [PubMed]
- Agaram NP, Shia J, Tang LH, et al. DNA mismatch repair deficiency in ampullary carcinoma: a morphologic and immunohistochemical study of 54 cases. Am J Clin Pathol 2010;133:772-80. [Crossref] [PubMed]
- Saetta AA. K-ras, p53 mutations, and microsatellite instability (MSI) in gallbladder cancer. J Surg Oncol 2006;93:644-9. [Crossref] [PubMed]
- Nagai M, Watanabe M, Iwase T, et al. Clinical and genetic analysis of noncancerous and cancerous biliary epithelium in patients with pancreaticobiliary maljunction. World J Surg 2002;26:91-8. [Crossref] [PubMed]
- Haswell-Elkins MR, Sithithaworn P, Elkins D. Opisthorchis viverrini and cholangiocarcinoma in Northeast Thailand. Parasitol Today 1992;8:86-9. [Crossref] [PubMed]
- Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248-57. [PubMed]
- Shia J, Tang LH, Vakiani E, et al. Immunohistochemistry as first-line screening for detecting colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: a 2-antibody panel may be as predictive as a 4-antibody panel. Am J Surg Pathol 2009;33:1639-45. [Crossref] [PubMed]
- Imai Y, Inoue T, Ishikawa T. Mutations of the human MUT S homologue 6 gene in ampullary carcinoma and gastric cancer. Int J Cancer 1998;78:576-80. [Crossref] [PubMed]
- Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer 1999;81:214-8. [Crossref] [PubMed]
- Barrow E, Robinson L, Alduaij W, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet 2009;75:141-9. [Crossref] [PubMed]
- Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012;12:237-51. [Crossref] [PubMed]


