Does hepatitis B virus infection cause breast cancer?
Introduction
In western countries breast cancer (BC) is one of the leading cause of cancer related death in women, whereas, its incidence is low in Chinese females. The studies of migrant populations have shown that when low-risk groups (e.g., Chinese groups) move to high-risk regions (Hawaii or mainland USA), their incidence of BC increases rapidly, approaching the rates of the host population within one or two generations (1,2). These marked differences in BC incidence suggest that environmental factors might have a major influence on the risk of developing BC; in fact, environmental factors might be more important than the influence of genetic factors, perhaps with the notable exception of the familial BC syndromes. So, what are the main prevalent and environmental carcinogenic factors to cause the early age onset of BC in China?
Several viruses have been implicated in the pathogenesis of a subset of BC (3). A population-based case-control study about association between chronic viral hepatitis infection and BC risk suggested that chronic hepatitis C virus (HCV) infection was associated with early-onset BC (4). Similarly, as a hepadnavirus, hepatitis B virus (HBV) infection is more common than HCV in China, where every surgical patient should undergo routine examination of HBV serological markers and liver function tests for peri-operative preparation before operation. Though with the nationwide vaccination program since 1992, the epidemiology of HBV infection in China is still moderate endemic now. Approximately 60% of the Chinese population have laboratory evidence of contact with HBV and 7.2% are chronic carriers of HBsAg (5). In this paper We propose that HBV infection might have a key role in the early development of BC in China (seen in Figures 1,2).
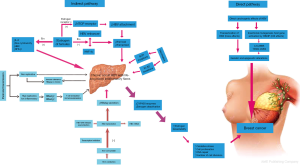

Biology of HBV infection
In general, the natural history of chronic HBV infection in birth or early childhood is divided into five phases as follows: high replicative low inflammatory, immune clearance, HBeAg (−) chronic, non-replicative, HBsAg loss/occult hepatitis B (6). It is important to note that these stages of infection are not static or always sequential, with possibility of moving from one phase to another in any direction. Viral replication and development of liver disease depend on the balance between viral mechanisms promoting persistence, and host immune control (7). Understanding of the timing of acquisition of the infectious agent in early life and cancer development later in life is unclear. HBV is acquired in early life, during in utero, infancy, early childhood, and adolescence, have a long latency period in human carcinogenesis, but this relationship of HBV with BC is largely unexplored yet.
The vast majority of acutely infected adults are able to spontaneously ‘‘clear’’ the virus from the blood (8). However, in most cases, those individuals who have cleared HBsAg still maintain a low level of infection throughout their lives, especially various cases have been reported of the persistence of HBV as occult infection in BC patients with frequent reactivation during immunosuppressive therapy (9). The, mutations in the pre-S/S genome region of HBV also results in reduced production of HBsAg (10). Apart from mutations within the HBV genome, there are other mechanisms that the virus may utilize to evade an immune response also further by infection of host immune cells, such as peripheral mononuclear cells, integration into the host genome, formation of HBV-containing immune complexes and modulating the host immune response directly (11). In addition to suppressing innate immune responses, the use of a transcriptional template [covalently closed circular DNA (cccDNA)] sequestered in the nucleus of infected cells may allow the virus to evade detection by the innate immune system (12). On the other hand, secreted HBeAg or even HBsAg are also proposed to be viral immunosuppressors to induce the exhaustion of helper T cells (13). Notably, HBV relies on retroviral replication strategy (reverse transcription from RNA to DNA), and eradication of HBV infection is rendered difficult because the stable, long enduring, cccDNA becomes established in hepatocyte nuclei and HBV DNA becomes integrated into the host genome (14,15). Surprisingly, HBV may retreat into immunologically privileged places from where it can seed the circulation and reach cytotoxic T lymphocytic (CTL) inaccessible tissues or even cause immunosupression, thereby maintaining the CTL response in apparently cured individuals and thus prolonging the liver disease to chronic HBV hepatitis (16).
In general, occult blood infection represents the window period of acute infection, persistence of low level replication after recovery, or the occurrence of an escape mutant undetected by current HBsAg assays (17). The occult HBV infection (OBI) prevalence in Asian population, is much higher and ranges from 7.5–16%. In several groups, the prevalence of OBI would be even greater if the tests were performed on liver tissue (18,19). Adult patients with HBV genotype C has been reported to achieve HBeAg seroconversion at an older age than genotype B (20). In China, HBV genotype C is highly prevalent which shows that Chinese females are more prone to late seroconversion and viral persistence when infected with HBV. Studies have shown that occult HBV may be transmitted between relatives or transmitted to children from occult infected or HBsAg-positive mothers (21,22). Additionally, recovery from an acute self-limited hepatitis B may also be followed by OBI, which could last greater than 2–3 decades (23). Hence, OBI must be more widespread in Chinese populace as HBV being endemic in China and it remains undetectable for decades.
Possible role of HBV as a carcinogenic factor for BC
The indirect role of HBV as a carcinogenic factor for BC
After family history, lifetime cumulative exposure to reproductive hormones, especially oestrogen, is the most important risk factor for BC. Longer a woman is exposed to cycling reproductive hormones, higher her risk of developing BC (24,25). Although HBV is considered as a non-direct cytopathic virus, persistent and prolonged HBV infection in the liver is accompanied by histological signs of mild hepatic necro inflammation (26), which leads to increase in the level of estrogen as its mainly deactivated in the liver. There have been number of studies on the role of estrogen in causing BC (27-30). Hence, increased exposure of free estrogen due to long term liver dysfunction may lead to breast carcinoma (seen in Figure 1).
HBV is considered to be a sex hormone responsive virus in essence, regardless of the sex disparity in host immune responses (31). ERα (an estrogen receptor) mediates in the transcriptional downregulation of NTCP gene (32) (NTCP is a major receptor for infectious entry of HBV). NTCP gene downregulation was confirmed to be 2-fold lower in female rats than in males (33). This might be one plausible mechanism for estrogen/ERα axis as an anti-HBV guardian to restrict viral infection or spread in liver tissues of females (34). The seroconversion from HBeAg to anti-HBe and from HBsAg to anti-HBs is also observed more frequently in females than in male subjects (35). Thus, for HBV infection, immune clearance of serum HBeAg and HBsAg is achieved in a higher percentage of female HBV patients than males, which shows that, estrogen paves HBV to remain in occult state and reactivate further with the return of favourable condition.
Estrogens are known to be potent repressors of IL-6 production (36). It can also repress transcription of HBV genes by up-regulating ER-α, which interacts with and alters binding of HNF-4α to the HBV enhancer (37). Thus, estrogen decreases viral load as well as inhibit immune system from attacking the virus. Hence with this delicate balancing action of estrogen by inhibiting virus and immune system leads to increased tendency of developing occult HBV in females.
ERα which is activated by estrogen in the persistently injured stage of liver, also restrains reactive oxygen species (ROS)-mediated cytotoxicity by inhibiting the activation of NF-κB, leading to the suppression of NADPH oxidase activity and decrement of ROS production (38). Due to the defensive effect of estrogen pathway the HBx ability to disturb the redox potential of mitochondrial transmembrane and enhance ROS generation was attenuated in females. These findings suggest that estradiol has a hepatoprotective effect with anti-inflammatory action, by inhibiting proinflammatory cytokines. Hence, long term exposure to estrogen in female HBV carriers was associated with a lower risk of HCC development (39,40). Conversely, it may promote early development of breast carcinoma with long term raise in estrogen levels due to its decreased deactivation thanks to decades of on/off chronic occult necro inflammatory damage to liver by HBV (seen in Figure 1).
A Taiwan study recently reported that HCV infection, instead of HBV infection, appeared to be associated with the risk of early onset of BC in areas endemic for HCV and HBV (4). Wong et al. (41) reported that screening all patients for HBsAg before adjuvant chemotherapy for BC would prevent a significant number of HBV reactivations, and would likely be moderately cost-effective, which might point to the fact that HBV infection and BC coexist more often and are closely related.
Meanwhile it is well known that a number of in vitro studies have clearly demonstrated that the HCV “core” protein strongly inhibits HBV replication (42) and OBI infection is commonly observed in patients coinfected with non-occult HCV (43). A study reported 50% of chronic HCV patients had previous exposure to HBV in the form of anti-HBc (44). Surprisingly, anti-HBc is well accepted as the most sensitive marker in evaluating HBV infection history. Nearly 100% of chronic HBV subjects and more than 90% of individuals with OBI are HBcAb positive (45,46). Also studies have shown that HCV RNA as a significant predictor for OBI (47), which clearly shows that HCV and HBV infection often coexists together. Furthermore, inhibition of HBV replication and reduction in HBsAg synthesis have been reported in coinfection with HCV (48). The underlining molecular mechanism responsible for this suppressive effect are being extensively studied both in vitro (49) and in vivo studies (50). Therefore, with this fact it must be noted that Taiwan’s clinical study have not included the occult HBV cases which might be present in large numbers of HCV infected patients. Henceforth, the difference in sensitivity and/or specificity of methods used in it could be responsible for the discrepant findings.
Possible role of HBV as a direct carcinogenic factor for BC
HBV-DNA integration into host genome occurs at early steps of clonal tumor expansion and induces both genomic instability and direct insertional mutagenesis of diverse cancer-related genes. HBV DNA is proved to be found in low concentration in the breast milk (51,52). It has also been detected in peripheral blood mononuclear cells (PBMC) and extrahepatic tissues such as, bone marrow cells, spleen, and lymphoblastoid cell lines (53). Also much evidence indicates that HBV may maintain its pro-oncogenic role even in occult infection (54).
The breast oncoprotein hepatitis B X-interacting protein (HBXIP), a conserved 18 KDa protein, is originally identified by its interaction with the hepatitis B virus X protein (HBX) which decreases HBV replication together with HBsAg and HBeAg synthesis, and is believed that the effect is mediated by inhibition of HBX action on the endogenous viral core promoter/enhancer elements (55). High HBXIP expression is predominantly observed in BC tissues instead of the adjacent normal breast tissues and plays a crucial role as a key oncoprotein in the development of BC (55-63). HBXIP also activates the transcriptional coregulatory protein, LIM-only protein 4 (LMO4) through transcription factor Sp1 to promote the proliferation of BC cells (60). It also enhances the angiogenesis and growth of BC cells through modulating fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) (64). It further enhances the growth and migration of BC cells through S100A4 in vivo and in vitro (58) and upregulates Lin28B, thus promoting BC (55) (seen in Figure 1).
Carcinoprotective factors in Chinese diet and breast carcinoma
Flavonoids are a family of polyphenolic compounds synthesized by plants with a similar structure, are divided into subclasses, including anthocyanidins, flavanones, flavonols, flavones and isoflavones (65). Soy phytoestrogens, such as genistein, daidzein, and glycitein, are isoflavonoids closely related to human 17β-estradiol (66), but with lower estrogenic activity (67). Several beneficial properties have been attributed to these dietary compounds, including antioxidant, anti-inflammatory, and anti-carcinogenic effects. Phytoestrogens such as genistein, resveratrol, bakuchiol and quercetin have anti-proliferative effects on BC cells (68,69). A favoured mechanism by which soy isoflavones may influence BC development is via their affinity and competition with endogenous oestrogens and other substrates in binding with oestrogen receptors (ERs). Surprisingly it was also reported that S-equol had a greater affinity for ER than its precursor isoflavone daidzein (70), which has a demethylating effect on the CpG islands in the promoters of BRCA1 and BRCA2 genes (71) thus suppressing familial type BRCA gene mutations related breast carcinoma.
It has also been reported that dietary intake of mushrooms and green tea decreased BC risk (72). It is found in vitro studies that tea polyphenols inhibit aromatase, the key enzyme converting androgens to estrone or estradiol (73) and is associated with reduced levels of estrogens, estrone, and estradiol among pre- and postmenopausal Chinese women (73,74). Henceforth it’s a proven fact that Chinese diet rich in soy, vegetables, green tea and fruits plays a carcinoprotective role, thus, reducing incidence of BC in Asia (75-77) (seen in Figure 2).
Stark imbalance in the exposure of carcinogenic and carcinoprotective factors leads to early development of BC
Breast tissue is highly dynamic throughout a woman’s life, and during any of these unique stages, a disruption in the delicate balance of growth factor and hormonal signaling between the stroma and epithelium has the potential to promote disease. The wide imbalance in the amount of exposure to carcinogenic factor (e.g., HBV infection) for decades which belittles the effect of carcinoprotective factors may lead to early development of BC (seen in Figure 2). Controversially, imbalance in the amount of exposure to carcinoprotective factors for decades which belittles the effect of carcinogenic factor (e.g., HBV infection) may lead to low incidence of BC in China.
Controversies regarding HBV vaccine efficacy and its possible consequences
HBV vaccine has led to significant reduction in viral transmission. The nationwide vaccination program since 1992 has changed the epidemiology of HBV infection in China from being highest to moderately endemic at present. Erstwhile, It is reported that the efficacy of vaccine is not 100% and is protective for at least 10 years (78). Studies from various countries have detected HBV chronic infections among immunized infants and children (79-83). Many factors may be related to occult infection in vaccinated children, such as hypo-response to HBV vaccination, declining antibody titers, escape mutations in S gene, or high maternal viral loads (84). Additionally, it seems that HBV infection risk is increased dramatically during adolescence. The cumulative 10-year HBV infection risk in postnatal passive active HBV vaccinated subjects was less than 15% (85). On the contrary, more than 30% of these high-risk subjects were HBcAb positive at 15 years of age (86) which indicates the possibility that, with increasing age HBV shows the trend in which it can retain undetected in the dormant state all along the normal efficacy of vaccine and reappear slowly into active state when the vaccine efficacy seems to fade away.
Conclusions
The seemingly conflicting phenomenon of early age onset and lower BC incidence in China might be due to wide imbalance in the amount of exposure to carcinogenic factor (e.g., HBV infection) for decades and the carcinoprotective exposure levels (e.g., isoflavonoids and flavonoids intake) (seen in Figures 1,2). In other words, the increase in carcinoprotective levels and decrease in carcinogenic levels would lead to lower incidence of breast cancer and vice versa. Thus understanding the role of chronic occult HBV and its relation with the estrogen could lead to new ways of prevention, diagnosis and adjuvant therapeutic treatments, and potentially benefit females by decreasing the incidence and increasing overall survival. Also, screening and monitoring of HBV and its vaccine efficacy should be enhanced from neonatal period, which can probably prevent or delay the occurrence of breast carcinoma.
Acknowledgements
We would like to thank Professor Kainan Wu from Chongqing Breast Cancer Center, Chongqing Medical University for his critical comments.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: The opinions and conclusions reported in this article are those of the authors.
References
- Trichopoulos D, Yen S, Brown J, et al. The effect of westernization on urine estrogens, frequency of ovulation, and breast cancer risk. A study of ethnic Chinese women in the Orient and the USA. Cancer 1984;53:187-92. [Crossref] [PubMed]
- Chen C, Sun S, Yuan JP, et al. Characteristics of breast cancer in Central China, literature review and comparison with USA. Breast 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Hennighausen L. Mouse models for breast cancer. Breast Cancer Res 2000;2:2-7. [Crossref] [PubMed]
- Su FH, Chang SN, Chen PC, et al. Association between chronic viral hepatitis infection and breast cancer risk: a nationwide population-based case-control study. BMC Cancer 2011;11:495. [Crossref] [PubMed]
- Ministry of Health, China. 2006–2010 National guidelines for hepatitis B prevention and treatment. 2006 January 28.
- Gish RG, Given BD, Lai CL, et al. Chronic hepatitis B: Virology, natural history, current management and a glimpse at future opportunities. Antiviral Res 2015;121:47-58. [Crossref] [PubMed]
- Seetharam A, Perrillo R, Gish R. Immunosuppression in Patients with Chronic Hepatitis B. Curr Hepatol Rep 2014;13:235-44. [Crossref] [PubMed]
- Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med 2004;350:1118-29. [Crossref] [PubMed]
- Arai M, Kobayashi J, Saito M, et al. Chemotherapy-Induced Reactivation of Hepatitis B in Recurrent Breast Cancer - A Case Report. Gan To Kagaku Ryoho 2015;42:1115-8. [PubMed]
- Katsoulidou A, Paraskevis D, Magiorkinis E, et al. Molecular characterization of occult hepatitis B cases in Greek blood donors. J Med Virol 2009;81:815-25. [Crossref] [PubMed]
- Hu KQ. Occult hepatitis B virus infection and its clinical implications. J Viral Hepat 2002;9:243-57. [Crossref] [PubMed]
- Ferrari C. HBV and the immune response. Liver International 2015;35:121-8. [Crossref] [PubMed]
- Chu CM, Liaw YF. Chronic hepatitis B virus infection acquired in childhood: special emphasis on prognostic and therapeutic implication of delayed HBeAg seroconversion. J Viral Hepat 2007;14:147-52. [Crossref] [PubMed]
- Seeger C, Ganem D, Varmus HE. Biochemical and genetic evidence for the hepatitis B virus replication strategy. Science 1986;232:477-84. [Crossref] [PubMed]
- Dienstag JL. Hepatitis B virus infection. N Engl J Med 2008;359:1486-500. [Crossref] [PubMed]
- Rehermann B, Fowler P, Sidney J, et al. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med 1995;181:1047-58. [Crossref] [PubMed]
- Reesink HW, Engelfriet CP, Henn G, et al. Occult hepatitis B infection in blood donors. Vox Sang 2008;94:153-66. [Crossref] [PubMed]
- Morales-Romero J, Vargas G, Garcia-Roman R. Occult HBV infection: a faceless enemy in liver cancer development. Viruses 2014;6:1590-611. [Crossref] [PubMed]
- Raimondo G, Navarra G, Mondello S, et al. Occult hepatitis B virus in liver tissue of individuals without hepatic disease. J Hepatol 2008;48:743-6. [Crossref] [PubMed]
- Livingston SE, Simonetti JP, Bulkow LR, et al. Clearance of Hepatitis B e Antigen in Patients With Chronic Hepatitis B and Genotypes A, B, C, D, and F. Gastroenterology 2007;133:1452-7. [Crossref] [PubMed]
- Datta S, Banerjee A, Chandra PK, et al. Genotype, phylogenetic analysis, and transmission pattern of occult hepatitis B virus (HBV) infection in families of asymptomatic HBsAg carriers. J Med Virol 2006;78:53-9. [Crossref] [PubMed]
- Walz A, Wirth S, Hucke J, et al. Vertical transmission of hepatitis B virus (HBV) from mothers negative for HBV surface antigen and positive for antibody to HBV core antigen. J Infect Dis 2009;200:1227-31. [Crossref] [PubMed]
- Bläckberg J, Kidd-Ljunggren K. Occult hepatitis B virus after acute self-limited infection persisting for 30 years without sequence variation. J Hepatol 2000;33:992-7. [Crossref] [PubMed]
- Hankinson SE, Eliassen AH. Endogenous estrogen, testosterone and progesterone levels in relation to breast cancer risk. J Steroid Biochem Mol Biol 2007;106:24-30. [Crossref] [PubMed]
- Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast Cancer Res 2007;9:R28. [Crossref] [PubMed]
- Raimondo G, Caccamo G, Filomia R, et al. Occult HBV infection. Semin Immunopathol 2013;35:39-52. [Crossref] [PubMed]
- Meilahn EN, De Stavola B, Allen DS, et al. Do urinary oestrogen metabolites predict breast cancer? Guernsey III cohort follow-up. Br J Cancer 1998;78:1250-5. [Crossref] [PubMed]
- Mackey RH, Fanelli TJ, Modugno F, et al. Hormone therapy, estrogen metabolism, and risk of breast cancer in the Women's Health Initiative Hormone Therapy Trial. Cancer Epidemiol Biomarkers Prev 2012;21:2022-32. [Crossref] [PubMed]
- Arslan AA, Koenig KL, Lenner P, et al. Circulating estrogen metabolites and risk of breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev 2014;23:1290-7. [Crossref] [PubMed]
- Ziegler RG, Fuhrman BJ, Moore SC, et al. Epidemiologic studies of estrogen metabolism and breast cancer. Steroids 2015;99:67-75. [Crossref] [PubMed]
- Tong S. Hepatitis B virus, a sex hormone-responsive virus. Gastroenterology 2012;142:696-9. [Crossref] [PubMed]
- Cao J, Wood M, Liu Y, et al. Estradiol represses prolactin-induced expression of Na+/taurocholate cotransporting polypeptide in liver cells through estrogen receptor-alpha and signal transducers and activators of transcription 5a. Endocrinology 2004;145:1739-49. [Crossref] [PubMed]
- Simon FR, Fortune J, Iwahashi M, et al. Characterization of the mechanisms involved in the gender differences in hepatic taurocholate uptake. Am J Physiol 1999;276:G556-65. [PubMed]
- Wang SH, Chen PJ, Yeh SH. Gender disparity in chronic hepatitis B: Mechanisms of sex hormones. J Gastroenterol Hepatol 2015;30:1237-45. [Crossref] [PubMed]
- Zacharakis GH, Koskinas J, Kotsiou S, et al. Natural history of chronic HBV infection: a cohort study with up to 12 years follow-up in North Greece (part of the Interreg I-II/EC-project). J Med Virol 2005;77:173-9. [Crossref] [PubMed]
- Ray P, Ghosh SK, Zhang DH, et al. Repression of interleukin-6 gene expression by 17 beta-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-kappa B by the estrogen receptor. FEBS Lett 1997;409:79-85. [Crossref] [PubMed]
- Wang SH, Yeh SH, Lin WH, et al. Estrogen receptor alpha represses transcription of HBV genes via interaction with hepatocyte nuclear factor 4alpha. Gastroenterology 2012;142:989-98.e4. [Crossref] [PubMed]
- Shimizu I, Kohno N, Tamaki K, et al. Female hepatology: favorable role of estrogen in chronic liver disease with hepatitis B virus infection. World J Gastroenterol 2007;13:4295-305. [Crossref] [PubMed]
- Yu MW, Chang HC, Chang SC, et al. Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology 2003;38:1393-400. [PubMed]
- Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007;317:121-4. [Crossref] [PubMed]
- Wong WW, Hicks LK, Tu HA, et al. Hepatitis B virus screening before adjuvant chemotherapy in patients with early-stage breast cancer: a cost-effectiveness analysis. Breast Cancer Res Treat 2015;151:639-52. [Crossref] [PubMed]
- Raimondo G, Cacciamo G, Saitta C. Hepatitis B virus and hepatitis C virus co-infection: additive players in chronic liver disease? Ann Hepatol 2005;4:100-6. [PubMed]
- Morsica G, Ancarani F, Bagaglio S, et al. Occult hepatitis B virus infection in a cohort of HIV-positive patients: correlation with hepatitis C virus coinfection, virological and immunological features. Infection 2009;37:445-9. [Crossref] [PubMed]
- Khan HA, Umar M, Hamama Tul B, et al. Effect of Previous Exposure to HBV on Liver Histology and Treatment Response in CHC Patients. J Coll Physicians Surg Pak 2015;25:498-500. [PubMed]
- European Association For The Study Of The Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012;57:167-85. [Crossref] [PubMed]
- Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection J Hepatol 2008;49:652-7. [Crossref] [PubMed]
- Said ZN, El-Sayed MH, El-Bishbishi IA, et al. High prevalence of occult hepatitis B in hepatitis C-infected Egyptian children with haematological disorders and malignancies. Liver Int 2009;29:518-24. [Crossref] [PubMed]
- Hollinger FB, Sood G. Occult hepatitis B virus infection: a covert operation. J Viral Hepat 2010;17:1-15. [Crossref] [PubMed]
- Bellecave P, Gouttenoire J, Gajer M, et al. Hepatitis B and C virus coinfection: a novel model system reveals the absence of direct viral interference. Hepatology 2009;50:46-55. [Crossref] [PubMed]
- Guido M, Thung SN, Fattovich G, et al. Intrahepatic expression of hepatitis B virus antigens: effect of hepatitis C virus infection. Mod Pathol 1999;12:599-603. [PubMed]
- Chen X, Chen J, Wen J, et al. Breastfeeding is not a risk factor for mother-to-child transmission of hepatitis B virus. PloS One 2013;8:e55303. [Crossref] [PubMed]
- Montoya-Ferrer A, Zorrilla AM, Viljoen J, et al. High Level of HBV DNA Virus in the Breast Milk Seems not to Contraindicate Breastfeeding. Mediterr J Hematol Infect Dis 2015;7:e2015042. [Crossref] [PubMed]
- Stoll-Becker S, Repp R, Glebe D, et al. Transcription of hepatitis B virus in peripheral blood mononuclear cells from persistently infected patients. J Virol 1997;71:5399-407. [PubMed]
- Pollicino T, Saitta C, Raimondo G. Hepatocellular carcinoma: the point of view of the hepatitis B virus. Carcinogenesis 2011;32:1122-32. [Crossref] [PubMed]
- Liu Q, Bai X, Li H, et al. The oncoprotein HBXIP upregulates Lin28B via activating TF II D to promote proliferation of breast cancer cells. Int J Cancer 2013;133:1310-22. [Crossref] [PubMed]
- Marusawa H, Matsuzawa S, Welsh K, et al. HBXIP functions as a cofactor of survivin in apoptosis suppression. EMBO J 2003;22:2729-40. [Crossref] [PubMed]
- Melegari M, Scaglioni PP, Wands JR. Cloning and characterization of a novel hepatitis B virus x binding protein that inhibits viral replication. J Virol 1998;72:1737-43. [PubMed]
- Liu S, Li L, Zhang Y, et al. The oncoprotein HBXIP uses two pathways to up-regulate S100A4 in promotion of growth and migration of breast cancer cells. J Biol Chem 2012;287:30228-39. [Crossref] [PubMed]
- Xu F, You X, Liu F, et al. The oncoprotein HBXIP up-regulates Skp2 via activating transcription factor E2F1 to promote proliferation of breast cancer cells. Cancer Lett 2013;333:124-32. [Crossref] [PubMed]
- Yue L, Li L, Liu F, et al. The oncoprotein HBXIP activates transcriptional coregulatory protein LMO4 via Sp1 to promote proliferation of breast cancer cells. Carcinogenesis 2013;34:927-35. [Crossref] [PubMed]
- Zhang Y, Zhao Y, Li L, et al. The oncoprotein HBXIP upregulates PDGFB via activating transcription factor Sp1 to promote the proliferation of breast cancer cells. Biochem Biophys Res Commun 2013;434:305-10. [Crossref] [PubMed]
- Cheng D, Liang B, Li Y. HBXIP expression predicts patient prognosis in breast cancer. Med Oncol 2014;31:210. [Crossref] [PubMed]
- Zhang Y, Zhao Y, Li H, et al. The nuclear import of oncoprotein hepatitis B X-interacting protein depends on interacting with c-Fos and phosphorylation of both proteins in breast cancer cells. J Biol Chem 2013;288:18961-74. [Crossref] [PubMed]
- Liu F, You X, Wang Y, et al. The oncoprotein HBXIP enhances angiogenesis and growth of breast cancer through modulating FGF8 and VEGF. Carcinogenesis 2014;35:1144-53. [Crossref] [PubMed]
- Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise Int J Oncol 2007;30:233-45. (review). [PubMed]
- Messina M, Hilakivi-Clarke L. Early intake appears to be the key to the proposed protective effects of soy intake against breast cancer. Nutr Cancer 2009;61:792-8. [Crossref] [PubMed]
- Dixon RA. Phytoestrogens. Annu Rev Plant Biol 2004;55:225-61. [Crossref] [PubMed]
- Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res 2010;27:962-78. [Crossref] [PubMed]
- Li L, Chen X, Liu CC, et al. Phytoestrogen Bakuchiol Exhibits In Vitro and In Vivo Anti-breast Cancer Effects by Inducing S Phase Arrest and Apoptosis. Front Pharmacol 2016;7:128. [Crossref] [PubMed]
- Muthyala RS, Ju YH, Sheng S, et al. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem 2004;12:1559-67. [Crossref] [PubMed]
- Bosviel R, Durif J, Dechelotte P, et al. Epigenetic modulation of BRCA1 and BRCA2 gene expression by equol in breast cancer cell lines. Br J Nutr 2012;108:1187-93. [Crossref] [PubMed]
- Zhang M, Huang J, Xie X, et al. Dietary intakes of mushrooms and green tea combine to reduce the risk of breast cancer in Chinese women. Int J Cancer 2009;124:1404-8. [Crossref] [PubMed]
- Kapiszewska M, Miskiewicz M, Ellison PT, et al. High tea consumption diminishes salivary 17beta-estradiol concentration in Polish women. Br J Nutr 2006;95:989-95. [Crossref] [PubMed]
- Wu AH, Arakawa K, Stanczyk FZ, et al. Tea and circulating estrogen levels in postmenopausal Chinese women in Singapore. Carcinogenesis 2005;26:976-80. [Crossref] [PubMed]
- Kang X, Zhang Q, Wang S, et al. Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. CMAJ 2010;182:1857-62. [Crossref] [PubMed]
- Cho YA, Kim J, Park KS, et al. Effect of dietary soy intake on breast cancer risk according to menopause and hormone receptor status. Eur J Clin Nutr 2010;64:924-32. [Crossref] [PubMed]
- Wang H, Tao L, Qi K, et al. Quercetin reverses tamoxifen resistance in breast cancer cells. J Buon 2015;20:707-13. [PubMed]
- McMahon BJ, Bruden DL, Petersen KM, et al. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Ann Intern Med 2005;142:333-41. [Crossref] [PubMed]
- Wang Z, Zhang J, Yang H, et al. Quantitative analysis of HBV DNA level and HBeAg titer in hepatitis B surface antigen positive mothers and their babies: HBeAg passage through the placenta and the rate of decay in babies. J Med Virol 2003;71:360-6. [Crossref] [PubMed]
- Shao ZJ, Zhang L, Xu JQ, et al. Mother-to-infant transmission of hepatitis B virus: a Chinese experience. J Med Virol 2011;83:791-5. [Crossref] [PubMed]
- Chen SJ, Zhao YX, Fang Y, et al. Viral deletions among healthy young Chinese adults with occult hepatitis B virus infection. Virus Res 2012;163:197-201. [Crossref] [PubMed]
- Yotsuyanagi H, Yasuda K, Iino S, et al. Persistent viremia after recovery from self-limited acute hepatitis B. Hepatology 1998;27:1377-82. [Crossref] [PubMed]
- Fukuda R, Ishimura N, Niigaki M, et al. Serologically silent hepatitis B virus coinfection in patients with hepatitis C virus-associated chronic liver disease: clinical and virological significance. J Med Virol 1999;58:201-7. [Crossref] [PubMed]
- Raimondo G, Pollicino T, Cacciola I, et al. Occult hepatitis B virus infection. J Hepatol 2007;46:160-70. [Crossref] [PubMed]
- Huang LM, Chiang BL, Lee CY, et al. Long-term response to hepatitis B vaccination and response to booster in children born to mothers with hepatitis B e antigen. Hepatology 1999;29:954-9. [Crossref] [PubMed]
- Lu CY, Chiang BL, Chi WK, et al. Waning immunity to plasma-derived hepatitis B vaccine and the need for boosters 15 years after neonatal vaccination. Hepatology 2004;40:1415-20. [Crossref] [PubMed]


