Adjuvant therapy for resected biliary tract cancer: a review
Introduction
Biliary tract cancer (BTC) encompasses cholangiocarcinoma of the intrahepatic, hilar and distal bile duct, as well as gallbladder cancer. There is significant global variation in the incidence of gallbladder cancer, for example in the UK where age-adjusted incidence rates are 0.4–0.6/100,000 compared with Delhi, India where incidence rates reach 21.5/100,000 women, and Chile where rates of 15.6/100,000 women are seen (1-3). In most countries, the incidence and mortality from gallbladder cancer is falling over time (2). The opposite is true for intrahepatic cholangiocarcinoma, which has seen a significant rise in incidence in recent years, particularly in the USA where rates have risen from 0.32/100,000 between 1975 and 1979 to 0.85/100,000 between 1995 and 1999 (4). Known risk factors for cholangiocarcinoma include primary sclerosing cholangitis, thorotrast exposure, recurrent fluke infections, congenital biliary abnormalities and hepatitis C infection (5-9). The primary risk factor for gallbladder cancer is gallstone disease, but there is also a recognized association with Peutz-Jeghers syndrome and Gardner’s syndrome (10-12). The molecular pathology of these diseases also appears to be heterogeneous, with KRAS mutations seen in intra- and extra-hepatic cholangiocarcinoma more frequently than gallbladder cancer, IDH mutation seen exclusively in intrahepatic cholangiocarcinoma, and PIK3CA mutations seen more frequently in gallbladder cancer (13,14).
Surgery is currently the only treatment which offers a hope of cure in this disease, but even those with localized disease have survival rates of 15–30% at 5 years from registry data, and outcomes from surgical series report 18–63% survival at 5 years (15-20). From one series, 5-year survival following surgery was 63% for intrahepatic, 30% for perihilar and 27% for distal tumors, demonstrating a significant difference based on tumor location (19). A second large series from Japan reported 5-year survival rates of 52.8% for peri-ampullary cancers, 41.6% for gallbladder cancer and 33.1% for bile duct tumors, again highlighting significant differences based on primary disease site (20). Tumor recurrence is frequent, and while many patients develop local recurrence, a significant number have distant metastatic disease at the time of relapse (21). From one series, 17% of patients with gallbladder cancer and 41% of patients with hilar cholangiocarcinoma (HC) developed local recurrence, and distant metastasis was seen in 72% and 36%, respectively. The pattern of relapse may be different depending on the site of the initial tumor, but overall these data provide the rationale to consider adjuvant treatment using chemotherapy or radiotherapy, potentially controlling both systemic and local recurrences. Currently, there is a lack of high quality published data supporting these treatments. Several retrospective series exist, as well as some prospective studies with limitations: inclusion of patients with other disease types (pancreatic or peri-ampullary carcinoma), small sample size, inclusion of patients with unresectable disease and a lack of standard surgical management. This review aims to summarize and interpret the available data supporting adjuvant therapy in this disease, as well as highlighting ongoing studies and avenues for future research.
Methodology
Literature searches of PubMed (1960 to July 2015), Embase (1980 to July 2015), American Society of Clinical Oncology (ASCO) annual meetings (2009 to 2015), ASCO Gastrointestinal Symposia (2009 to 2015) and European Society of Medical Oncology Congresses (2009 to 2015) were performed. Searches were limited to English language publications and human studies. No lower patient limit was applied. Published studies were excluded if the full text was unavailable. The main keywords used were cholangiocarcinoma, biliary cancer, gallbladder cancer, adjuvant, neoadjuvant, chemotherapy, radiotherapy, chemoradiotherapy. Citation lists of retrieved articles were also manually reviewed. Guideline documents were obtained from their respective sources.
Adjuvant radiotherapy
In the setting of a disease characterized by local recurrence in many cases as well as surgical approaches that are often technically difficult, adjuvant radiotherapy has been adopted by many centers for patients with BTC. The role of radiotherapy as a sole adjuvant modality of treatment has not been studied in a prospective clinical trial, and given the effect of systemic therapy it is unlikely that any future study is planned. As such, the only data regarding the relative effectiveness of radiotherapy comes from retrospective series and population-based registry analyses. Retrospective series have reported improvements in median survival from 8 to 24 months, improvements in 5-year survival from 13.5% to 33.9% using adjuvant radiotherapy or have failed to demonstrate improvement in survival, but these have often been limited by small numbers and a lack of standardized treatment, as well as the inclusion of patients with advanced disease who did not truly have radical resection (22-24). The results of these series are summarized in Table 1, along with studies of adjuvant chemotherapy and chemoradiotherapy.
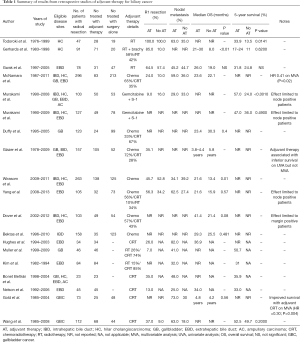
Full table
Analyses of data from the US Surveillance, Epidemiology, and End Results (SEER) database form the basis of the largest studies to examine the effect of adjuvant radiotherapy in gallbladder cancer and intrahepatic cholangiocarcinoma. From the report by Hyder et al., 5,011 patients with intrahepatic cholangiocarcinoma were identified between 1988 and 2009 (25). Radical surgery was performed in 3,619; 1,842 had a limited resection. Radiotherapy was delivered in 899 patients: these were more likely to be young, more recently diagnosed, have higher grade and more extensive disease (positive lymph nodes, higher T stage) than those who did not receive radiotherapy. No information was available regarding systemic chemotherapy administration. On unmatched survival analysis, there appeared to be an early overall survival (OS) advantage in those who received adjuvant radiotherapy over no radiotherapy (1-year OS 68.2% vs. 58%, P=0.03), while later landmark survival analysis at 5 years showed the opposite effect (5-year OS 20.2% vs. 28%, P=0.04). Due to the imbalances in prognostic features between groups, a propensity matched survival analysis was undertaken using 894 patients treated with surgery alone who had similar clinicopathological features to those treated with adjuvant radiotherapy. The results of this analysis were in favor of adjuvant radiotherapy, with median OS in the adjuvant therapy group measured at 18 months compared with 11 months for patients treated with surgery alone (HR =0.45; P<0.001).
The SEER analysis of adjuvant radiotherapy in resected gallbladder cancer was reported by Wang et al. in 2007, and formed the basis for a nomogram for predicting the benefit of this treatment (26). Between 1988 and 2003, 4,180 patients met the study inclusion criteria. Patients were included across all stages, and 881 patients with metastatic disease were included in the analysis. Of the total population, 760 received radiotherapy, and 3,420 had surgical resection without radiotherapy. No information was reported regarding extent of surgical resection, and no information was available on the use of adjuvant or concurrent chemotherapy. Patients receiving radiotherapy had more locally advanced disease and nodal metastasis but fewer had distant metastatic disease. On unadjusted analysis of OS, patients who received radiotherapy had longer median survival than those treated with surgery alone (15 vs. 8 months, P<0.0001). A survival model was developed using the multivariate regression, and this estimated no survival benefit from the addition of adjuvant radiotherapy in patients with T1 tumors. An estimated survival benefit was seen in patients with T2 or higher tumors, or those with nodal involvement.
These population-based analyses using SEER data have the advantage of large numbers of patients, but contain a mix of patients with resected and metastatic disease, and the lack of information regarding chemotherapy administration limits the conclusions that can be drawn regarding the utility of adjuvant radiotherapy.
Adjuvant chemotherapy
Chemotherapy has been used for several years in advanced disease, but only with the publication of the ABC-02 study in 2010 has there been consensus regarding the optimal regimen (27). Prior to this, a number of non-randomized phase II studies and underpowered phase III studies had formed the evidence base for chemotherapy regimens such as gemcitabine, 5-fluorouracil (5-FU), or platinum combinations in unresectable or metastatic disease (28). With this background, it is unsurprising that there is a lack of standardized therapy in studies of adjuvant chemotherapy.
Prospective data regarding the efficacy of adjuvant chemotherapy in resected BTC is limited. A Japanese phase III study of adjuvant chemotherapy in patients with pancreatobiliary cancer was reported in 2002, and remains the only prospective randomized trial published regarding this treatment (29). This study enrolled 173 patients with pancreatic cancer, 139 with bile duct cancer, 140 with gallbladder cancer and 56 with ampullary cancer. Patients were randomized to chemotherapy with mitomycin C and 5-FU (MF) or surgery alone. In a per-protocol analysis of patients with gallbladder cancer, 5-year survival was significantly better for the MF group than the surgery alone group (26% vs. 14.4%, P=0.0367), however this difference was no longer statistically significant in an intention-to-treat analysis (P=0.2819). The 5-year disease-free survival (DFS) rate in patients with gallbladder cancer was also higher in the MF group than controls (20.3% vs. 11.6%, P=0.021). There were no significant differences between 5-year survival rates for MF or controls in patients with pancreatic (11.5% vs. 18%), bile duct (26.7% vs. 24.1%) or ampullary (28.1% vs. 34.3%) cancers. This study had some serious methodological issues: patients undergoing curative and non-curative resections were included, along with a large and imbalanced number of ineligible patients, making it difficult to draw definitive conclusions based on these results.
The ESPAC-3 study was a prospective study of adjuvant chemotherapy (5-FU with folinic acid or gemcitabine) compared with observation alone after curative surgery in 434 patients with ampullary cancer, peri-ampullary duodenal cancer, or intrapancreatic bile duct cancer (30). The primary OS results for the entire cohort of patients did not demonstrate a significant benefit for 5-FU (HR =0.95; P=0.74) or gemcitabine (HR =0.77; P=0.1) over observation. Results from secondary analyses adjusting for prognostic variables demonstrated improved survival for patients treated with any chemotherapy (HR =0.75; P=0.03) or gemcitabine (HR =0.70; P=0.03). Significant differences were seen in survival based on tumor location in this study: patients with ampullary cancer had a median survival time of 53.1 months compared with 20.9 months for those intrapancreatic bile duct tumors and 32.6 months for those with other tumors, reflecting the different biology of these tumors. Results for patients with each tumor type based on treatment received are summarized in Table 2. No statistically significant difference in survival in response to treatment was observed in this study between patients with pancreatobiliary or intestinal subtypes of ampullary cancer. Taken as a whole, these data provide modest support for adjuvant chemotherapy in ampullary cancer, but cannot be extrapolated to other biliary cancer types.

Full table
A number of retrospective studies form the majority of data regarding adjuvant chemotherapy, all of which are limited by the lack of standardized adjuvant protocols and surgical approaches, as well as selection bias. One of the largest single institution series comes from Princess Margaret Cancer Centre, published by McNamara et al. (31). This examined 296 patients treated with definitive surgery for biliary cancer between 1987 and 2011. In this group, 83 received adjuvant therapy (5-FU- or gemcitabine-based chemotherapy or chemoradiotherapy) and 213 had surgery alone, and those who received adjuvant therapy had significantly improved OS (HR =0.60; P=0.03). There were imbalances between the groups: more patients in the adjuvant therapy group had R1 resection (24% vs. 10%, P=0.001) and nodal involvement (59% vs. 25%, P<0.0001), and the adjuvant therapy group contained more patients with distal bile duct cancer (53% vs. 36%), and fewer with intrahepatic tumors (7% vs. 21%, P=0.02 for tumor location). Adjuvant therapy was associated with improved OS in multivariable analysis (MVA) (HR =0.41; P=0.02), with median OS of 23.6 months for patients treated with adjuvant therapy compared with 22.1 months for surgery alone. Patients with older age and nodal metastases had significantly shorter survival on multivariable analysis.
Another large retrospective series, detailing the experience with adjuvant gemcitabine and S-1 chemotherapy after curative intent surgery in a Japanese center, was the subject of publications in 2009 and 2011 (32,33). The first study included 103 patients with gallbladder cancer, ampullary cancer and cholangiocarcinoma (50 treated with chemotherapy and 53 with surgery alone), while the second included 127 patients with hilar, intra- or extra-hepatic cholangiocarcinoma (49 treated with chemotherapy and 78 with surgery alone). In both reports, adjuvant chemotherapy was associated with better 5-year OS rates (57% vs. 24%, P<0.001 and 47% vs. 36%, P=0.49, respectively). From the 2009 paper, there were no significant clinicopathological differences between those treated with adjuvant chemotherapy and those treated with surgery alone. In this group, the survival advantage for adjuvant chemotherapy was seen primarily in the node-positive group (n=53, P=0.16) and not in patients with node-negative disease (n=74, P=0.393).
A third large institutional dataset, from Memorial Sloan Kettering Cancer Centre, was the subject of a publication by Duffy et al. in 2008 (34). This study included 123 patients who underwent curative surgery with negative margins for gallbladder cancer between 1995 and 2005. Twenty-four patients received adjuvant therapy: 8 with chemoradiotherapy, 8 with chemotherapy (5-FU or gemcitabine), and 8 with chemotherapy following chemoradiotherapy. Median survival for these patients was 23.4 months, compared with 30.3 months for those treated with surgery alone (P=0.4). A significant number of the patients treated with adjuvant therapy had positive lymph nodes (11/24), or either positive surgical margins or resected metastases (8/24) compared with the surgery alone arm.
Similar results were reported by Glazer et al. in a series of 157 patients treated with curative surgical resection for gallbladder cancer or cholangiocarcinoma between 1978 and 2009 at MD Anderson Cancer Centre (35). Of these, 52 were treated with surgery alone and had median OS of 5.8 years. A cohort of patients (n=28) had neoadjuvant gemcitabine-based chemotherapy, which was associated with a trend toward increased hazard of death on univariate analysis (UVA) (HR =1.66; P=0.07). A second cohort (n=53) treated with adjuvant chemotherapy had an increased hazard of death on UVA (HR =1.69; P=0.04). A third group (n=24) treated with adjuvant gemcitabine-based chemoradiotherapy trended toward an increased hazard of death (HR =1.14; P=0.71). The median OS for patients treated with neoadjuvant or adjuvant chemotherapy was 3.8 years, and for those treated with chemoradiotherapy was 4.4 years. On MVA, none of the adjuvant or neoadjuvant therapies were associated with an effect on OS. This report does not contain a comparison of baseline factors between these groups, leaving open the possibility that those treated with adjuvant therapies had high-risk features such as nodal metastases, higher tumor grade or R1 resection. Another recent report from Alabama was presented by Dover et al. in 2014 (36). From one center, 103 patients underwent radical resection of cholangiocarcinoma. Of these, 49 had adjuvant chemotherapy or chemoradiotherapy. The overall analysis showed a non-significant trend to improved OS in patients treated with adjuvant therapy (median OS 41.4 vs. 21.4 months, P=0.08). When the analysis was limited to patients with R1 resections, adjuvant therapy was associated with a significant OS benefit compared with surgery alone (median OS 28.4 vs. 19.4 months, P=0.036). No details were provided regarding the adjuvant treatment protocols, and there was no comparison of baseline patient characteristics or known prognostic factors, limiting the interpretation of these results.
Recently, a report from authors Wirasorn et al. was published concerning adjuvant chemotherapy in a cohort from Thailand (37). This study included 263 patients with cholangiocarcinoma (intrahepatic, perihilar or distal bile duct) treated with curative-intent surgery. Of these, 125 were treated with surgery alone, and 138 received postoperative gemcitabine- or 5-FU-based chemotherapy regimens. The group receiving chemotherapy were younger than the surgery alone group (mean age 57.7 vs. 60.4 years, P=0.01), and fewer had serum albumin <3 g/dL (11.6% vs. 20.8%, P=0.04), but otherwise there were no significant differences in baseline characteristics including tumor site or stage, nodal stage or margin status. Patients who were treated with adjuvant chemotherapy had longer median OS than those treated with surgery alone (21.6 vs. 13.4 months, P=0.01). Additional analysis accounting for prognostic factors showed the greatest effect in patients with T4 tumors, positive lymph nodes, elevated CA19-9 levels, positive surgical resection margins and AJCC stage IV disease. In total, 27% of the patients included in this study had stage IV disease (a similar number were included in each group), and approximately half had positive surgical resection margins.
The most recent retrospective institutional dataset was reported by Yang et al. from a large Chinese center (38). This study included 105 patients with extrahepatic cholangiocarcinoma who underwent radical surgical resection, of whom 32 were treated with adjuvant chemotherapy or chemoradiotherapy. Similar to several other studies, patients treated with adjuvant therapy were younger (90.6% vs. 69.9% under 70 years, P=0.02), but were more likely to have T3/4 tumors (46.9% vs. 24.7%, P=0.02), nodal metastasis (62.5% vs. 27.4%, P=0.001) and R1 resections (56.3% vs. 34.2%, P=0.035) than those treated with surgery alone. In the overall analysis, there was no evidence of a survival benefit in those treated with adjuvant therapy (HR =0.87; P=0.57). When patients with nodal metastasis were analyzed, there was evidence of better median OS in those treated with adjuvant therapy compared with surgery alone (21.6 vs. 10.4 months, P=0.02). On MVA, nodal metastasis (HR =2.19; P=0.009), R1 resection (HR =1.89; P=0.015) and adjuvant therapy (HR =0.45; P=0.011) were associated with OS.
Additional data regarding adjuvant chemotherapy in gallbladder cancer comes from a registry analysis of 4,774 patients treated in Japan (39). Surgical resection was undertaken in 3,324 patients, and 74% had complete macroscopic resection. Chemotherapy was used as adjuvant therapy in 36% of men and 38% of women. Overall, the 5-year survival rate was higher in patients who had surgery alone compared with those who received adjuvant chemotherapy (33% vs. 45%, P<0.01). This may be explained by an imbalance in staging between groups: rates of chemotherapy use were 23–25% for patients with stage I disease and 43–53% for those with stage IV disease, suggesting that patients receiving chemotherapy were more likely to have more advanced disease. When survival rates were compared stage by stage between chemotherapy and surgery alone, patients who received chemotherapy and had stage IVA disease had a higher 5-year survival rate (14% vs. 12%, P<0.05). No significant differences were seen in other stage groups. Details regarding clinicopathological features in this dataset are limited to stage, age and gender, and no there is no information regarding which chemotherapy regimens were used. As such, interpretation of these data is difficult.
Some evidence, therefore, exists to support the use of adjuvant chemotherapy in patients with gallbladder cancer, extrahepatic cholangiocarcinoma and ampullary tumors. Data supporting adjuvant therapy in intrahepatic cholangiocarcinoma are more limited. In 2014, a systematic review of retrospective series of patients with intrahepatic cholangiocarcinoma was reported by Mavros et al. (40). Of the 57 studies included, 14 examined the effect of chemotherapy, transarterial chemoembolization or radiotherapy as adjuvant therapy (n=2,289). Neither adjuvant chemotherapy nor chemoradiotherapy were associated with survival benefit in any of the nine studies that examined these treatments. Radiotherapy alone was analyzed in two studies and appeared to be beneficial in one. Unfortunately, the authors did not report a meta-analysis or pooled analysis of these results. A separate report in 2014 from Hanover, Germany included 158 patients treated with surgical resection for intrahepatic cholangiocarcinoma (41). Of these, 35 were treated with adjuvant chemotherapy (consisting of gemcitabine in 90.9%). Irrespective of tumor stage or nodal involvement, there was a trend to improvement in median OS for those treated with adjuvant chemotherapy compared with surgery alone but no statistically significant difference was seen (29.3 vs. 25.5 months, P=0.481).
Chemoradiotherapy
An adjuvant approach combining systemic chemotherapy with local radiotherapy has been adopted by some groups in an attempt to combine the local control effect of radiotherapy with adjuvant chemotherapy. Prospective evidence for this approach in extrahepatic cholangiocarcinoma and gallbladder cancer has recently come from the phase II SWOG S0809 study, described later (42). As background to this study, a number of retrospective series have reported benefit for adjuvant chemoradiotherapy in biliary cancer—predominantly extrahepatic and gallbladder tumors. Many of these are small series reporting patients treated with this approach, without comparison against groups treated with surgery alone (43-47). These reported survival rates of 13–51% at 5 years, but feature a broad spread of disease stages, surgical techniques and chemoradiotherapy regimens. In general, the regimens used combined external beam radiotherapy at doses of ≥45 gray (Gy) with 5-FU based chemotherapy delivered over 5 weeks. The results of the retrospective studies of adjuvant chemoradiotherapy are summarized in Table 2.
One of the larger single-institution retrospective series was reported by Hughes et al. in 2007 (43). This group contained 34 patients treated with adjuvant chemoradiotherapy following pancreato-duodenectomy for stage II/III distal bile duct tumors at Johns Hopkins Hospital over a 10 years period, and the results were compared with 30 historical controls treated at the same institution. The median dose of radiotherapy was 50.4 Gy, and the concurrent chemotherapy used was 5-FU-based, in combination with mitomycin C, interferon or cisplatin and followed by maintenance 5-FU. In total, 26% of patients had an R1 resection and 82% had nodal metastases. Patients treated with adjuvant chemoradiotherapy had longer median OS when compared with historical controls (20 vs. 8 months, P<0.04), in spite of a lower rate of nodal involvement or positive surgical margins in the controls. These data are clearly limited by the small number and lack of contemporaneous controls, but suggested a benefit in this population.
In 2009, Gold et al. described the outcomes of patients treated at the Mayo Clinic using adjuvant chemoradiotherapy (48). Between 1985 and 2004, 73 patients underwent surgical resection of stage I/II gallbladder cancer with negative surgical margins. Forty-eight patients had no adjuvant therapy, and 25 were treated with chemoradiotherapy: external beam radiotherapy at a median dose of 50.4 Gy in 28 fractions with concurrent bolus 5-FU during week 1 and 5 of treatment. Patients receiving adjuvant therapy were more likely to have stage II disease (P<0.001), nodal metastasis (P<0.001) or T3 tumors (P<0.001). The median OS was 4.2 years for patients treated with surgery alone, and 4.8 years for those who received adjuvant therapy (log-rank P=0.56). When the prognostic factors of T stage and nodal status were incorporated into a multivariate model, receipt of adjuvant therapy was predictive of improved OS (HR =0.30; P=0.004).
A series of patients treated with adjuvant chemoradiotherapy for gallbladder cancer from a combined multi-institutional US dataset was reported this year by Wang et al. (49). Across six major centers, 112 patients underwent radical resection, and 68 of these were treated with adjuvant chemoradiotherapy. Limited information was provided on chemotherapy, but only five patients were treated with radiotherapy alone. More patients treated with chemoradiotherapy had T3–4 tumors (57% vs. 16%, P<0.01), R1 resections (37% vs. 9%) and nodal metastasis (63% vs. 18%, P<0.01), but this group were also younger (median age 60 vs. 68 years, P=0.01). OS for the whole cohort was 50.6% at 5 years. In the overall analysis, no significant difference was seen in 5-year OS between those treated with adjuvant therapy and surgery alone (49.7% vs. 52.5%, P=0.20). On multivariate analysis, nodal metastasis (HR =3.52; P<0.01) and more recent surgery (HR =0.21; P<0.01) were significantly associated with OS, while no significant effect from adjuvant therapy was seen (HR =0.78; P=0.51). This relatively large retrospective series of gallbladder cancer patients showed no significant improvement in survival for those treated with adjuvant chemoradiotherapy, but the imbalances in major prognostic factors limit the interpretation of these results.
A registry analysis of patients included in the SEER-Medicare database treated with chemoradiotherapy for resected gallbladder cancer was published by Wang et al. in 2011, and formed the basis for developing a nomogram aiming to help make individualized survival estimates (50). One thousand and one hundred thirty-seven patients were included in this study, including 885 treated with surgical resection alone, 126 treated with adjuvant chemotherapy and 126 treated with adjuvant chemoradiotherapy. Patients receiving chemoradiotherapy were younger and more likely to have advanced T stage or nodal metastasis than those treated with surgery alone. On MVA, both adjuvant chemotherapy (P=0.034) and adjuvant chemoradiotherapy (P<0.001) were associated with better survival. In a similar fashion to these authors’ previous publication on radiotherapy, a nomogram was developed which suggested that the benefit from chemoradiotherapy was more substantial than the benefit seen from adjuvant chemotherapy, particularly in patients with more advanced disease. At the time of writing, this nomogram is available online: http://skynet.ohsu.edu/nomograms/postcrt/gallbladder.html.
To date, the most influential data regarding adjuvant chemoradiotherapy or chemotherapy has come from a meta-analysis of published studies by Horgan et al. in 2012 (51). This included data from many of the above mentioned retrospective institutional series, registry analyses and prospective studies. Eligible trials included patients with intrahepatic and extrahepatic cholangiocarcinoma as well as gallbladder cancer; those with ampullary tumors were excluded. Adjuvant therapy consisted of chemotherapy, radiotherapy or both. In total, 20 studies were eligible for inclusion, containing 6,712 patients: 4,915 treated with surgery alone and 1,797 treated with adjuvant therapy. Of these studies, 1 was a randomized trial, 2 were registry analyses and 17 were institutional series. Only one study included patients with intrahepatic cholangiocarcinoma, and this was a small cohort (n=11). Six studies focused on gallbladder cancer, and 14 dealt with bile duct tumors. In the overall analysis, adjuvant therapy was associated with a trend toward improvement in survival (OR =0.74; P=0.06). On sensitivity analysis, there was evidence of greater benefit for chemotherapy (OR =0.39; P<0.001) and chemoradiotherapy (OR =0.61; P=0.049) than radiotherapy (OR =0.98; P=0.90). Further pooled analyses were carried out on nine studies which reported nodal and surgical margin status. Patients (n=230) with nodal metastasis were found to derive significant benefit from adjuvant therapy (OR =0.49; P=0.004), and a similar advantage was seen in 216 patients who had R1 resections (OR =0.36; P=0.002). Patients with R1 resections appeared to derive a benefit from adjuvant radiotherapy (OR =0.33; P=0.01); this was not seen in patients with clear surgical margins (OR =1.26; P=0.20). There are obvious limitations to this analysis: published data rather than individual patient data were used, the majority of the studies included are retrospective series which are susceptible to selection bias, there is significant variation in the adjuvant therapy delivered across the studies, better staging techniques may have resulted in stage migration over the time period covered by the studies [1962–2008] and publication bias may be a factor with the possibility of negative results being under-reported. In spite of these failings, many centers use this study as a basis for recommending adjuvant therapy in patients with high-risk biliary cancer.
As mentioned above, the SWOG S0809 study is a prospective phase II study which has recently reported its final results (42). Given the data has previously been almost exclusively confined to extrahepatic cholangiocarcinoma and gallbladder cancer, these were the populations included in this trial. Patients were eligible if they had resected T2–4 or N1 tumors, or had positive surgical margins. Adjuvant therapy consisted of four cycles of chemotherapy with gemcitabine and capecitabine, followed (in the absence of disease progression) by radiotherapy with concurrent capecitabine (45–59.4 Gy to surgical bed and 45 Gy to regional lymph nodes). A comprehensive quality assurance program was in place for reviewing surgery, pathology and radiotherapy plans. Across 12 centers, 105 patients were registered between 2008 and 2012. After central review, 21 were deemed ineligible, leaving 79 for analysis. The primary site was bile duct in 54 patients, and gallbladder in 25. Resection margins were clear in 54 patients, and 25 had microscopic positive margins. Of the 79 patients treated with chemotherapy, 69 patients (87%) went on to receive radiotherapy. In total, 68 (86%) patients were able to complete treatment as planned, with 3 discontinuations due to toxicity. With a median follow-up time of 35 months, 41 (42%) patients had died. Two-year OS was estimated at 65% for the whole group. No significant differences in 2-year OS were noted based on tumor site or margin status: estimates were 68% for patients with bile duct tumors, 56% for those with gallbladder tumors, 67% for those with R0 resection and 60% for those with R1 resection. DFS at 2 years was estimated to be 52% overall. Local recurrence occurred in 14 patients, 9 of whom also had synchronous distant relapse, and 24 patients had distant-only relapse.
This study showed that modern chemoradiotherapy techniques are safe, feasible to deliver and can produce survival rates significantly higher than those seen in historical controls. Accruing patients to a prospective clinical study in this disease is a challenge given its relatively low incidence and difficulty in its management, as can be evidenced by the lack of prospective data. The study is clearly limited by its lack of a control group as well as the inability to determine the relative benefits of chemotherapy or radiotherapy, but the authors correctly conclude that these results demonstrate the feasibility of conducting a multi-center trial in this disease and that chemoradiotherapy is a promising adjuvant regimen in gallbladder cancer and extrahepatic cholangiocarcinoma.
Published guidelines on adjuvant therapy
The evidence remains uncertain surrounding the benefit of adjuvant therapy in resected biliary cancer, and as such the guideline documents cannot make strong recommendations. At present, the National Comprehensive Cancer Network (NCCN) Guidelines for intrahepatic cholangiocarcinoma suggest that adjuvant chemotherapy may be an option for patients with negative or positive surgical margins, or lymph node metastasis, and that adjuvant chemoradiotherapy is an option for those with R1 resection or nodal involvement. For gallbladder cancer and extrahepatic cholangiocarcinoma, the guidelines suggest adjuvant chemotherapy or chemoradiotherapy are options for all patients with resected tumors, but that limited data exist, no standard regimen can be recommended and clinical trial participation is encouraged (52). The European Society for Medical Oncology (ESMO) Clinical Practice Guidelines for biliary cancer suggest that in light of high incidence of local failure after surgical resection, postoperative fluoropyrimidine- or gemcitabine-based chemoradiotherapy may be considered based on the results of retrospective studies (53). The European Association for the Study of the Liver (EASL) guidelines for the management of intrahepatic cholangiocarcinoma were updated in 2014, and state that there is no established adjuvant therapy after surgical resection, but that adjuvant therapy should be strongly considered, especially in those with nodal metastasis (54). The British Society of Gastroenterology (BSG) guidelines for treatment of cholangiocarcinoma state that there is no current evidence to support the use of adjuvant chemotherapy or radiotherapy, and restate the need for appropriate clinical trials (55).
Ongoing clinical trials and future directions
There are currently three phase III studies of adjuvant chemotherapy in biliary cancer whose results are awaited. The BILCAP study from the Cancer Research UK Clinical Trials Group (NCT00363584) commenced in 2006, and closed to recruitment in 2013 after enrolling an estimated 360 patients (56). This study randomized patients with resected biliary cancer (gallbladder cancer, intrahepatic, hilar and extrahepatic cholangiocarcinoma) to adjuvant chemotherapy with capecitabine or observation. The primary endpoint is 5-year OS, and results are expected soon. The French PRODIGE-12 study (NCT01313377) opened to accrual in 2009, and randomized patients with resected biliary cancer to adjuvant chemotherapy with gemcitabine and oxaliplatin or observation (57). Its estimated enrolment is 190 patients and this is expected to be reported in 2017. The ACTICCA-1 study from the German, Dutch and British groups (NCT02170090) is currently recruiting patients with resected biliary cancer and involves random assignment to adjuvant chemotherapy with cisplatin and gemcitabine or observation (58,59). The primary endpoint is DFS, and an estimated 280 patients with cholangiocarcinoma and 80 patients with gallbladder cancer will be enrolled by an estimated completion date of April 2019. Tissue is being collected as part of the study for translational research to evaluate blood and tissue markers that may be prognostic or predictive of benefit from adjuvant chemotherapy. These ongoing studies are summarized in Table 3. At present, no studies of adjuvant chemoradiotherapy are ongoing. Given the encouraging results from the SWOG S0809 study, it is expected that a larger randomized study of an adjuvant chemo-radiation protocol vs. chemotherapy alone will be planned in the near future. Success will rely on cooperation of large trial groups, and international support.

Full table
Little progress has been made in the pursuit of targeted therapies in biliary cancer. To date, efforts to identify molecular targets through next-generation sequencing have resulted in only limited potential opportunities (13,14). Given the lack of efficacy data for any molecularly targeted therapy in advanced disease, it would be premature to expect these treatments to appear in adjuvant studies. However, it is vital that planned adjuvant trials include tissue collection protocols, annotated to clinical outcome, to allow the search for potential biomarkers using evolving knowledge and technologies.
Conclusions
Biliary cancer encompasses a biologically diverse spectrum of tumors, with notably disparate prognoses and patterns of recurrence and progression. In an already uncommon disease, this diversity has made study of new treatments extremely challenging. With the broad acceptance of chemotherapy as an active treatment since the publication of the ABC-02 study, adjuvant chemotherapy has been considered by many physicians in patients with high risk of recurrence, even in the absence of convincing prospective data. Similarly, with the observation of high local failure rates, adjuvant radiotherapy is frequently offered. As summarized in this review, several large cancer centers have reported improved survival in retrospective series of patients treated with these adjuvant approaches, and a meta-analysis of the published data has suggested a benefit in those with high-risk features (nodal metastasis or positive surgical margins). The results of ongoing phase III studies of adjuvant chemotherapy are eagerly awaited, and may form the basis for stronger recommendations for adjuvant therapy to improve outcomes in this disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: JJ Knox receives research support from Pfizer Inc., MK Doherty has no conflicts of interest to declare.
References
- Lazcano-Ponce EC, Miquel JF, Muñoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin 2001;51:349-64. [Crossref] [PubMed]
- Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer 2006;118:1591-602. [Crossref] [PubMed]
- Andia ME, Hsing AW, Andreotti G, et al. Geographic variation of gallbladder cancer mortality and risk factors in Chile: a population-based ecologic study. Int J Cancer 2008;123:1411-6. [Crossref] [PubMed]
- Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472-7. [Crossref] [PubMed]
- El-Serag HB, Engels EA, Landgren O, et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology 2009;49:116-23. [Crossref] [PubMed]
- Bergquist A, Ekbom A, Olsson R, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol 2002;36:321-7. [Crossref] [PubMed]
- van Kaick G, Dalheimer A, Hornik S, et al. The german thorotrast study: recent results and assessment of risks. Radiat Res 1999;152:S64-71. [Crossref] [PubMed]
- Srivatanakul P, Ohshima H, Khlat M, et al. Endogenous nitrosamines and liver fluke as risk factors for cholangiocarcinoma in Thailand. IARC Sci Publ 1991.88-95. [PubMed]
- De Kerckhove L, De Meyer M, Verbaandert C, et al. The place of liver transplantation in Caroli's disease and syndrome. Transpl Int 2006;19:381-8. [Crossref] [PubMed]
- Walsh N, Qizilbash A, Banerjee R, et al. Biliary neoplasia in Gardner's syndrome. Arch Pathol Lab Med 1987;111:76-7. [PubMed]
- Hsing AW, Gao YT, Han TQ, et al. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer 2007;97:1577-82. [Crossref] [PubMed]
- Wada K, Tanaka M, Yamaguchi K, et al. Carcinoma and polyps of the gallbladder associated with Peutz-Jeghers syndrome. Dig Dis Sci 1987;32:943-6. [Crossref] [PubMed]
- Chiu JW, Serra S, Kamel-Reid S, et al. Next-generation sequencing: Profiling gallbladder cancer (GBC). J Clin Oncol 2015;33:abstr 286.
- Ross JS, Wang K, Javle MM, et al. Comprehensive genomic profiling of biliary tract cancers to reveal tumor-specific differences and frequency of clinically relevant genomic alterations. J Clin Oncol 2015;33:abstr 4009.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2012. Available online: http://seer.cancer.gov/csr/1975_2012/
- Reddy SK, Clary BM. Surgical management of gallbladder cancer. Surg Oncol Clin N Am 2009;18:307-24. ix. [Crossref] [PubMed]
- Wade TP, Prasad CN, Virgo KS, et al. Experience with distal bile duct cancers in U.S. Veterans Affairs hospitals: 1987-1991. J Surg Oncol 1997;64:242-5. [Crossref] [PubMed]
- Hasegawa S, Ikai I, Fujii H, et al. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg 2007;31:1256-63. [Crossref] [PubMed]
- DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007;245:755-62. [Crossref] [PubMed]
- Miyakawa S, Ishihara S, Horiguchi A, et al. Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg 2009;16:1-7. [Crossref] [PubMed]
- Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer 2003;98:1689-700. [Crossref] [PubMed]
- Todoroki T, Ohara K, Kawamoto T, et al. Benefits of adjuvant radiotherapy after radical resection of locally advanced main hepatic duct carcinoma. Int J Radiat Oncol Biol Phys 2000;46:581-7. [Crossref] [PubMed]
- Gerhards MF, van Gulik TM, González González D, et al. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg 2003;27:173-9. [PubMed]
- Gwak HK, Kim WC, Kim HJ, et al. Extrahepatic bile duct cancers: surgery alone versus surgery plus postoperative radiation therapy. Int J Radiat Oncol Biol Phys 2010;78:194-8. [Crossref] [PubMed]
- Hyder O, Dodson RM, Sachs T, et al. Impact of adjuvant external beam radiotherapy on survival in surgically resected gallbladder adenocarcinoma: a propensity score-matched Surveillance, Epidemiology, and End Results analysis. Surgery 2014;155:85-93. [Crossref] [PubMed]
- Wang SJ, Fuller CD, Kim JS, et al. Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer. J Clin Oncol 2008;26:2112-7. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist 2008;13:415-23. [Crossref] [PubMed]
- Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 2002;95:1685-95. [Crossref] [PubMed]
- Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA 2012;308:147-56. [Crossref] [PubMed]
- McNamara MG, Walter T, Horgan AM, et al. Outcome of adjuvant therapy in biliary tract cancers. Am J Clin Oncol 2015;38:382-7. [Crossref] [PubMed]
- Murakami Y, Uemura K, Sudo T, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol 2011;18:651-8. [Crossref] [PubMed]
- Murakami Y, Uemura K, Sudo T, et al. Adjuvant gemcitabine plus S-1 chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann Surg 2009;250:950-6. [Crossref] [PubMed]
- Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan Kettering Cancer Centre (MSKCC). J Surg Oncol 2008;98:485-9. [Crossref] [PubMed]
- Glazer ES, Liu P, Abdalla EK, et al. Neither neoadjuvant nor adjuvant therapy increases survival after biliary tract cancer resection with wide negative margins. J Gastrointest Surg 2012;16:1666-71. [Crossref] [PubMed]
- Dover L, Jacob R, Wang T, et al. Impact of adjuvant therapies on survival in patients with cholangiocarcinoma. J Clin Oncol 2014;32:abstr 360.
- Wirasorn K, Ngamprasertchai T, Khuntikeo N, et al. Adjuvant chemotherapy in resectable cholangiocarcinoma patients. J Gastroenterol Hepatol 2013;28:1885-91. [Crossref] [PubMed]
- Yang H, Zhou J, Wei X, et al. Survival outcomes and progonostic factors of extrahepatic cholangiocarcinoma patients following surgical resection: Adjuvant therapy is a favorable prognostic factor. Mol Clin Oncol 2014;2:1069-75. [PubMed]
- Kayahara M, Nagakawa T. Recent trends of gallbladder cancer in Japan: an analysis of 4,770 patients. Cancer 2007;110:572-80. [Crossref] [PubMed]
- Mavros MN, Economopoulos KP, Alexiou VG, et al. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg 2014;149:565-74. [Crossref] [PubMed]
- Bektas H, Yeyrek C, Kleine M, et al. Surgical treatment for intrahepatic cholangiocarcinoma in Europe: a single center experience. J Hepatobiliary Pancreat Sci 2015;22:131-7. [Crossref] [PubMed]
- Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol 2015;33:2617-22. [Crossref] [PubMed]
- Hughes MA, Frassica DA, Yeo CJ, et al. Adjuvant concurrent chemoradiation for adenocarcinoma of the distal common bile duct. Int J Radiat Oncol Biol Phys 2007;68:178-82. [Crossref] [PubMed]
- Müller B, Sola JA, Carcamo M, et al. Adjuvant chemoradiation for resected gallbladder cancer: Treatment strategies for one of the leading causes of cancer death in Chilean women. Indian J Cancer 2013;50:184-8. [Crossref] [PubMed]
- Kim S, Kim SW, Bang YJ, et al. Role of postoperative radiotherapy in the management of extrahepatic bile duct cancer. Int J Radiat Oncol Biol Phys 2002;54:414-9. [Crossref] [PubMed]
- Bonet Beltrán M, Roth AD, Mentha G, et al. Adjuvant radio-chemotherapy for extrahepatic biliary tract cancers. BMC Cancer 2011;11:267. [Crossref] [PubMed]
- Nelson JW, Ghafoori AP, Willett CG, et al. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys 2009;73:148-53. [Crossref] [PubMed]
- Gold DG, Miller RC, Haddock MG, et al. Adjuvant therapy for gallbladder carcinoma: the Mayo Clinic Experience. Int J Radiat Oncol Biol Phys 2009;75:150-5. [Crossref] [PubMed]
- Wang J, Narang AK, Sugar EA, et al. Evaluation of Adjuvant Radiation Therapy for Resected Gallbladder Carcinoma: A Multi-institutional Experience. Ann Surg Oncol 2015;22 Suppl 3:S1100-6. [Crossref] [PubMed]
- Wang SJ, Lemieux A, Kalpathy-Cramer J, et al. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol 2011;29:4627-32. [Crossref] [PubMed]
- Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40. [Crossref] [PubMed]
- Benson AB 3rd, Abrams TA, Ben-Josef E, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw 2009;7:350-91. [PubMed]
- Eckel F, Brunner T, Jelic S, et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2011;22 Suppl 6:vi40-4. [Crossref] [PubMed]
- Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89. [Crossref] [PubMed]
- Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 2012;61:1657-69. [Crossref] [PubMed]
- ClinicalTrials.gov. A service of the U.S. National Institutes of Health. Capecitabine or Observation after Surgery in Treating Patients With Biliary Tract Cancer. Available online: http://ClinicalTrials.gov/show/NCT00363584
- ClinicalTrials.gov. A service of the U.S. National Institutes of Health. Gemcitabine Hydrochloride and Oxaliplatin or Observation in Treating Patients with Biliary Tract Cancer That Has Been Removed by Surgery. Available online: http://ClinicalTrials.gov/show/NCT01313377
- Stein A, Arnold D, Bridgewater J, et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer 2015;15:564. [Crossref] [PubMed]
- ClinicalTrials.gov. A service of the U.S. National Institutes of Health. Adjuvant Chemotherapy With Gemcitabine and Cisplatin Compared to Observation After Curative Intent Resection of Biliary Tract Cancer (ACTICCA-1). Available online: http://ClinicalTrials.gov/show/NCT02170090


