Controversies in proton therapy for prostate cancer
Introduction
Interest in proton therapy (PT) in the management of prostate cancer has steadily increased over the past two decades. Protons are charged particles that produce a significantly different dose distribution when compared to photon based radiation therapy techniques, including intensity-modulated radiation therapy (IMRT). PT reduces integral dose of radiation delivered to organs at risk (OARs) surrounding the target (1-3). This may translate into lower rates of gastrointestinal (GI) and genitourinary (GU) toxicities in patients being treated for prostate cancer. PT may also reduce the risk for second cancer development and sexual dysfunction when compared to IMRT (4).
Over the past decade, a number of new proton centers have opened across the United States, and many have promoted the use of PT in the management of prostate cancer. Since PT is more expensive than other radiation therapy techniques, like brachytherapy and IMRT (5), physicians, patients, and other stakeholders are interested in high-quality evidence supporting PT in the management of prostate cancer. Specifically, the medical community has called for comparative effectiveness studies to determine whether PT improves the therapeutic ratio in the management of prostate cancer when compared to other less-expensive treatment modalities.
This review will summarize the dosimetric advantages of PT compared with photon-based conventional radiation techniques (such as IMRT), its cost, cost effectiveness, and supporting literature for PT in the management of prostate cancer.
Potential advantages and disadvantages of PT
The potential advantages and disadvantages of PT result from its dosimetric features. Protons travel only a finite distance, proportional to their speed, which can be controlled during the acceleration process; they deposit most of their energy at the end of their range in an area called the “Bragg peak” and, unlike photon-based radiation, there is no exit dose beyond the target. Additionally, the entrance dose is relatively low when compared to external-beam photon radiation. For many target locations, these features reduce the excess radiation dose delivered to surrounding organs and the number of beams needed to treat a target when compared to photon radiation (Figure 1). Multiple dosimetric comparisons have been performed evaluating the extent of the dosimetric improvements that PT offers for prostate cancer and, in general, they have shown that PT reduces the dose of excess radiation provided to OARs in the low-to-moderate range [<50 gray (Gy) relative biological effectiveness (RBE)].
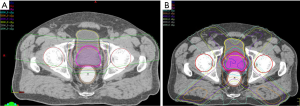
Trofimov et al. (2) published a comparison of passive-scattering PT, intensity-modulated PT (IMPT), and IMRT for the treatment of prostate cancer. The authors created comparison plans for ten patients treated for localized prostate cancer and found that PT reduced the V30 Gy(RBE) significantly to the rectum and bladder when compared to IMRT. Mean doses to the rectum also were reduced by 26% for PT when compared to IMRT. Furthermore, the PT plans provided better dose homogeneity than the IMRT plans as PT reduced the maximum dose and the volume receiving more than 110% of the max dose. PT also reduced the total irradiated volume of normal tissue in patients when compared with IMRT. Similarly, Vargas et al. (3) compared IMRT and passive-scattering PT treatment plans for ten patients with low-risk prostate cancer. PT significantly reduced all dose volumes from V10 to V80 Gy(RBE) to the rectum and rectal wall when compared to IMRT. All bladder volumes between V10 and V35 Gy(RBE) also were reduced significantly by PT when compared to IMRT. Finally, Chera et al. (1) evaluated IMRT and passive-scattering PT plans for a patient with high-risk prostate cancer who required pelvic nodal irradiation. The passive-scattering PT plans significantly reduced the dose of excess radiation to the rectum, rectal wall, bladder, and small bowel. Specifically, PT reduced the rectum V5 to V40 Gy(RBE) by 53% to 71% (P<0.05) when compared to IMRT. PT also reduced the V5 to V40 Gy(RBE) to the bladder by 40% to 63% (P<0.05). Because PT reduces the integral dose of excess radiation delivered to the body when compared to IMRT, PT will likely reduce the risk for secondary cancer development in patients surviving more than 10 years after treatment. Yoon et al. (4) compared the amount of scattered dose from PT to that from IMRT plans when treating prostate cancer. Scattered dose was an order of magnitude higher for the IMRT plans than for the PT plans, leading the authors to predict that IMRT was 5 times more likely to lead to secondary cancer development than PT. In a similar study, Fontenot et al. (6) found that the scattered dose from PT used to treat prostate cancer was much lower than that delivered by the IMRT plans. PT was predicted to reduce the risk for second malignancy after radiation therapy by 26% to 39% when compared to IMRT.
Nichols et al. (7) retrospectively reviewed the medical records of 171 men treated with PT for localized prostate cancer and found that PT does not affect serum testosterone levels. PT provides less excess radiation dose to the testes when compared to photon-based radiation. Consequently, while photon-based radiation lowers testosterone by 9% to 27% on average, patients treated with PT can expect no changes in testosterone, which may reduce the risk for fatigue or sexual dysfunction after treatment (7-9).
While PT reliably reduces dose to the bladder, rectum, and testes at lower dose ranges [<50 Gy(RBE)] irrespective of PT technique, potential dosimetric and radiobiologic disadvantages at higher doses [>50 Gy (RBE)] may occur depending on proton delivery method.
For example, Trofimov et al. (2) found that IMRT provided better dose conformality than passive-scattering PT. The average conformality index was 2.73 with IMRT and 3.11 with passive-scattering PT plans (P=0.004). The authors also found that doses to OARs in the high-dose range [>50 Gy(RBE)] were the same or worse with passive-scattering PT when compared to IMRT. For example, the average bladder V60 was 30% lower for the IMRT plans compared to the passive-scattering PT. On the other hand, when PT was delivered with intensity modulation with active scanning rather than passive scattering, IMPT provided better proximal dose conformality, reduced the lateral penumbra, and provided a better conformality index than IMRT. Underwood et al. had similar findings when comparing IMRT to passive-scattering PT (10). The authors found that IMRT provided lower doses in the range of 50 to 70 Gy(RBE) for both the rectum and the bladder when compared to passive-scattering PT.
Another potential disadvantage of PT is uncertainty in the RBE of PT compared with photon radiation. The effectiveness of the beam in causing cellular damage may be heterogeneous across the beam profile making predictions of tumor response and toxicity difficult. Although most clinical centers assume that PT has a constant RBE of 1.1, several investigators have found that the RBE along the proton beam path is not constant and often depends on beam depth (11,12). In vitro data suggest that the RBE is greater than 1.1 at the distal edge of the spread-out Bragg peak, and it may be higher at other beam locations as well (11,12). If ignored, variations in RBE may potentially place patients at higher risk for toxicity than photon radiation depending on the beam angles chosen, intrafraction motion, and target location (10). The variability of the RBE of protons can also be interpreted as a relative strength of PT. Some authors have called for the use of anterior beam orientations when treating prostate cancer so that the distal end of the spread-out Bragg peak is deposited on the peripheral zone of the prostate where most prostate cancers are found. The potentially higher RBE in this location could theoretically aid in tumor control (10).
Literature review
Non-comparative cohort studies
A few prospective and retrospective cohort studies have been published documenting the safety and efficacy of PT (Table 1) (13-21). In general, the studies have shown that PT minimizes the risk for major toxicity despite the use of dose escalation. PT also provides excellent biochemical control with overall rates of freedom from biochemical failure exceeding 90% in most studies featuring dose-escalated PT. In a study including patients treated to moderate doses of radiation therapy, Slater et al. published the results of a retrospective review of 1,255 patients treated with PT for localized prostate cancer between 1991 and 1997 (14). The median prescribed dose was 74 Gy(RBE) delivered at 2 Gy(RBE) per fraction. The median follow-up was 63 months; the overall 5- and 8-year biochemical control rates (BCRs) were 75% and 73%. Two cases of late grade 3 rectal bleeding and a case of grade 3 bowel obstruction requiring a colostomy were observed. Fourteen patients developed late grade 3 GU toxicity. The actuarial 5- and 10-year rates of freedom from late grade 3+ GI and GU toxicity was 99%.
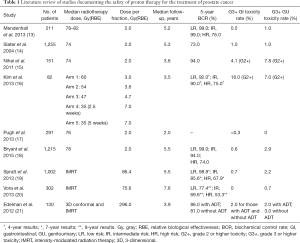
Full table
Mendenhall et al. (13) reported 5-year outcomes in three prospective trials featuring dose-escalated PT for patients with low-, intermediate- and high-risk prostate cancer, respectively. Patients were treated using passive-scattering PT to 78 to 82 Gy(RBE). The median follow-up was 5.2 years and the 5-year rates of freedom from biochemical failure for patients with low-, intermediate-, and high-risk prostate cancer were 99%, 99%, and 76%, respectively. Toxicity was recorded per the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0, guidelines and the rates of grade 3 GU and GI toxicity were 1.0% and 0.5%, respectively. Between baseline and >4 years of follow-up, patient-reported quality of life (QOL) did not significantly change according to the Expanded Prostate Cancer Index Composite (EPIC) sexual summary, bowel, or urinary irritative/obstructive summary scores. The same group of authors published an update of outcomes for a larger series of patients treated on the aforementioned protocols and enrolled on an outcomes-tracking protocol between 2006 to 2010 (18). Per the outcome-tracking protocol, the investigators collected patient-reported QOL and toxicity follow-up information prospectively. At 5 years, the rates of freedom from biochemical failure for patients with low-, intermediate-, and high-risk disease were 99%, 94%, and 74%. The 5-year rates of grade 3 GU and GI toxicity were 2.9% and 0.6%, respectively, per CTCAE, version 4. Patient-reported QOL significantly declined only within the EPIC sexual summary domain.
Nihei et al. (15) published a prospective multi-institutional phase II study evaluating the efficacy and safety of PT for localized prostate cancer. A total of 151 patients treated with passive-scattering PT to 74 Gy(RBE) at 2 Gy(RBE) per fraction were included in the analysis. The primary endpoint was late toxicity graded according to the CTCAE, version 2.0. With a median follow-up of 3.6 years, the rates of grade 2 GU and GI toxicity were 7.8% and 4.1%, respectively. The rate of overall freedom from biochemical failure was 94%.
Finally, Pugh et al. (17) published results of a prospective study including 226 men treated for localized prostate cancer with PT. With a minimum follow-up of 2 years, the incidence of grade 2+ GU and GI toxicity were 13.4% and 9.6%, respectively. Only a single patient experienced a grade 3 GI toxicity and no episodes of grade 3 GU toxicity were reported. The only meaningful decrement of patient-reported QOL following PT was in the bowel domain of the EPIC summary between baseline and >2 years of follow-up.
When compared to similar studies featuring IMRT for prostate cancer, PT compares favorably in terms of biochemical control while the risk of GI and GU toxicity is similar, as Table 1 shows. For example, Vora et al. (20) published results from a retrospective series of patients treated with high-dose photon radiation from the Mayo Clinic (Phoenix, AZ). Patients were treated to a median dose of 75.6 Gy. The median follow-up was for 7.6 years and the 9-year freedom from biochemical relapse rates were 77.4% for low-risk, 69.6% for intermediate-risk, and 53.3% for high-risk prostate cancer. The risk for late grade 3+ GI and GU toxicity was 0% and 0.7%, respectively. Additionally, Spratt et al. (19) published results from a series including 1,002 men with localized prostate cancer treated with high-dose IMRT at Memorial Sloan-Kettering Cancer Center (New York, NY, USA) between 1997 and 2008. The median dose delivered to the prostate was 86.4 Gy and the 7-year biochemical relapse-free survival rate was 98.8% for low-risk, 85.6% for intermediate-risk, and 67.9% for high-risk patients. The risk for late grade 3+ GI and GU toxicity was 0.7% and 2.2%, respectively.
Comparative studies of patient-reported QOL and toxicity
A few retrospective comparisons have been published evaluating toxicity and patient-reported QOL for patients treated with photon or proton beam radiation. The results have been mixed with some studies finding that PT reduces the risk for acute side effects of radiation therapy, as well as bowel frequency and urgency in comparison to photon based radiation, while other studies suggest that PT yields equivalent or worse outcomes. For example, Gray et al. evaluated 3-dimensional (3D) conformal photon radiation, IMRT, and PT using prospectively collected patient-reported QOL data (22). In the first 3 months of follow-up, the authors reported worse patient-reported QOL in the bowel/rectal, urinary irritative/obstruction, and incontinence domains for patients treated with 3D conformal radiotherapy and IMRT when compared to patients treated with PT; however, by 12 and 24 months of follow-up, no significant differences in patient-reported QOL were seen among the three groups.
Hoppe et al. (23) compared prospectively collected patient-reported QOL data for patients treated with PT for localized prostate cancer with patient-reported QOL from the Prostate Cancer Outcomes and Satisfaction (PROSTQA) treatment assessment study, which included men with prostate cancer treated with high-dose photon radiation. After 2 years of follow-up, there were no differences in EPIC bowel, urinary irritative/obstructive, or sexual summary scores between patients managed with PT or those managed with photon-based radiation. When confounding factors were accounted for, however, patients treated with IMRT had significantly more “moderate” or “big problems” with rectal urgency and bowel frequency than those treated with PT.
Fang et al. (24) published a case-matched study performed at the University of Pennsylvania (Philadelphia, PA, USA) comparing physician-reported toxicity for patients treated with IMRT or PT for localized prostate cancer. Patients in each group were retrospectively matched based on risk group, age, and prior GU and GI comorbidities. Acute GI toxicity was significantly lower among patients receiving PT than those receiving IMRT. By 1 and 2 years of follow-up, though, there was no significant difference in physician-reported grade 2+ GU or GI toxicity between IMRT and PT.
Finally, two studies using Medicare-based claims data extracted from the Surveillance, Epidemiology, and End Results (SEER) database have been published that compared toxicity for patients with prostate cancer treated with IMRT or PT. Sheets et al. (25) published the results of a study featuring 12,976 patients treated between 2000 and 2009 with IMRT, PT, or 3D conformal photon-based radiation therapy. In a propensity score-adjusted analysis, patients treated with PT did not differ in the risk for erectile dysfunction or urinary incontinence when compared to patients treated with IMRT. The authors found that PT was correlated with a higher risk for GI morbidity and GI procedures than IMRT. Yu et al. (26) published a Medicare-based comparative study of claims data including 27,647 patients treated with radiation therapy for prostate cancer. Patients treated with PT were matched to a subset of patients with similar socioeconomic and clinical characteristics who were treated with IMRT. The acute GU toxicity rate within the first 6 months of follow-up was significantly lower for patients who received PT than those who received IMRT. The risk for GI toxicity was not significantly different at any time during follow-up; by 12 months GU toxicity rates were also not significantly different between the two groups.
Because each comparison was made retrospectively and each study featured short follow-up, it is difficult to interpret the results of these comparative studies. The two SEER-based studies used Medicare reports as surrogates for toxicity. Consequently, all toxicities that did not have a Medicare code or did not lead to a procedure were not reported, which is a glaring weakness as their toxicity results are likely not comprehensive. Furthermore, both studies failed to report disease control data although it is possible that PT and IMRT have unaccounted-for differences in relative biologic effectiveness depending on the PT delivery method. Additionally, although patient-reported QOL data were provided in Fang et al. (24) and Gray et al. (22), the authors did not report differences in bowel frequency, diarrhea, or erectile dysfunction between the two treatment groups (22). This omission is important since the dosimetric improvements provided by PT result from reduced excess radiation in the low-to-moderate dose range, which may result in lower rates of erectile dysfunction, diarrhea, and bowel urgency, but not necessarily less rectal bleeding or urethral stricture. Like the aforementioned Medicare based studies, Fang et al. (24) and Gray et al. (22) also failed to report disease control rates, which may not be equivalent (5). Finally, some side effects, like long-term changes in bowel and bladder function and the development of second malignancies, require longer observation time to accurately measure the impact of PT.
Cost effectiveness
Today, PT is more expensive than other radiation therapy techniques. The construction and maintenance fees of a proton center increases the cost associated with the delivery of PT. Manufacturers of PT systems charge between $20 million (U.S.) for a one-room system and $200 million (U.S.) for a multi-room system for construction and installation, which is much higher than the costs of a similarly-sized photon-based radiation facility and equipment (26-30). Additionally, the operating costs of a proton center are estimated to be higher than those of a conventional photon-based radiation facility (31). Consequently, it is estimated that a course of standard fractionated PT (8 to 9 weeks of treatment) costs approximately $13,000 to $29,000 more per patient than IMRT (26-30). The higher cost associated with PT has drawn attention from stakeholders who seek to understand the potential benefits of PT and to determine if it is cost-effective.
Multiple authors have attempted to determine whether PT is cost-effective in the management of prostate cancer and the authors of each study relied on varying assumptions about the potential benefit of PT compared to IMRT and/or brachytherapy. Consequently, the estimates for cost-effectiveness for PT vary widely, raising questions about their validity. For example, Lundkvist et al. (31) published the results of a Markov model that showed that PT was cost-effective when compared to conventional radiation for prostate cancer. The authors assumed that PT would reduce the risk for prostate cancer recurrence by 20% when compared to IMRT if it was delivered at a higher dose, which is feasible because of better patient tolerability related to less dose to OARs. The authors also assumed that PT would reduce the risk for adverse events by 40%. With the criteria for cost-effectiveness set at 55,000 Euros per quality-adjusted life-year (QALY), PT was found to be cost-effective as its cost per QALY was only 26,800 Euros (31).
Conversely, Konski et al. (28) published the results of a Markov model used to assess the cost difference and QALY difference in patients treated with PT compared to IMRT for prostate cancer. The model included the assumption that PT could be used to escalate the dose to the prostate and improve cure rates while maintaining a similar toxicity profile as lower-dose IMRT. The authors found that the incremental cost-effectiveness ratio (ICER) for a >70-year-old patient was $63,578/QALY at 15 years of follow-up. The ICER for PT was shown to improve with longer follow-up, but never met the criteria for cost-effectiveness. The probability of cost-effectiveness was only 49% at 15 years. The ICER for a 60-year-old patient was $55,726/QALY at 15 years of follow-up after PT, which also did not meet the requirements for cost-effectiveness.
These cost-effectiveness estimates are problematic because both studies were performed before most of the prospective data supporting the efficacy and safety of PT was published (28,31). Additionally, these estimates may have changed since the cost of installing PT systems has decreased over time, potentially making PT more cost effective (26-29,32). Owing to these factors, estimates of the cost-effectiveness of PT will require continual adjustment as more data emerge and the cost of PT changes with time.
The future of PT
Currently, many stakeholders believe there is insufficient evidence to support PT in the management of prostate cancer. Still, the medical community can look forward to results from the PARTIQoL randomized trial that is currently accruing. This trial includes men with localized prostate cancer randomized to high-dose PT or IMRT. The primary outcome will be patient-reported QOL measured by EPIC mean bowel scores at 24 months of follow- up. The results of this trial may shed light on the relative benefit that PT could provide patients with prostate cancer. Nevertheless, because its patient cohort includes fewer than 500 men, the trial may not have enough power to detect clinically important differences in patient-reported bowel QOL. Additionally, bowel summary score may not be the best endpoint to study as it includes several symptoms, like rectal bleeding, bowel urgency, and bowel frequency, that commonly occur after radiation therapy but likely occur at different dose-volume thresholds. For example, rectal bleeding has been associated with the volume of rectal wall or rectum receiving doses of 75 to 78 Gy or higher in both the photon (33) and proton experiences (34), while bowel urgency and frequency, appear to be associated with the volume of rectum receiving doses of 40 Gy (23). Since the dose distributions between IMRT and PT are very different, advantages for one or the other may be obscured in the summary score, though apparent in individual symptom scores. Unfortunately, this randomized trial will likely not have enough power to evaluate each pertinent symptom within the EPIC bowel summary score individually, which is a potential weakness. Currently this randomized study between IMRT and PT is the only ongoing prospective comparative clinical trial, but it is likely that a much larger prospective comparative study will be necessary to determine whether PT has advantages for reducing specific toxicities and improving specific patient-reported QOL outcomes and/or improving disease control compared to IMRT in the management of localized prostate cancer.
In the future, improvements in the delivery of PT, including the adoption of IMPT and a reduction in the upfront costs of the development of proton facilities, are likely to improve the cost-effectiveness of PT. The technological improvement in the delivery of PT may amplify any outcome differences between PT and photon-based therapies. Consequently, the continued study of the relative benefits of PT must be performed to capture its true value.
Conclusions
In summary, PT remains a promising treatment for patients with prostate cancer because it provides less excess radiation to the OARs surrounding the prostate when compared to photon-based radiation therapy. Less excess dose to OARs is likely to result in improved toxicity rates and QOL, but also opens the door for escalation and/or intensification of dose to the prostate and potentially better disease control. Prospective studies support the safety and efficacy of PT in the management of prostate cancer; therefore, its use in these patients should not be considered experimental. Early prospective benchmark studies and retrospective comparative studies suggest that PT may reduce the risk for acute physician-reported GI and GU side effects and patient-reported acute and late bowel frequency and urgency and potentially improve disease control. Nevertheless, prospective comparative studies with extensive follow-up are needed to provide an accurate estimation of the relative benefits of PT as compared to IMRT and other prostate cancer treatments such as brachytherapy and surgery. Furthermore, as the costs associated with PT decrease, all cost-effectiveness comparisons will require re-evaluation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chera BS, Vargas C, Morris CG, et al. Dosimetric study of pelvic proton radiotherapy for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2009;75:994-1002. [Crossref] [PubMed]
- Trofimov A, Nguyen PL, Coen JJ, et al. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: a treatment planning comparison. Int J Radiat Oncol Biol Phys 2007;69:444-53. [Crossref] [PubMed]
- Vargas C, Fryer A, Mahajan C, et al. Dose-volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:744-51. [Crossref] [PubMed]
- Yoon M, Ahn SH, Kim J, et al. Radiation-induced cancers from modern radiotherapy techniques: intensity-modulated radiotherapy versus proton therapy. Int J Radiat Oncol Biol Phys 2010;77:1477-85. [Crossref] [PubMed]
- Hoppe BS, Bryant C, Sandler HM. Radiation for prostate cancer: intensity modulated radiation therapy versus proton beam. J Urol 2015;193:1089-91. [Crossref] [PubMed]
- Fontenot JD, Lee AK, Newhauser WD. Risk of secondary malignant neoplasms from proton therapy and intensity-modulated x-ray therapy for early-stage prostate cancer. Int J Radiat Oncol Biol Phys 2009;74:616-22. [Crossref] [PubMed]
- Nichols RC Jr, Morris CG, Hoppe BS, et al. Proton radiotherapy for prostate cancer is not associated with post-treatment testosterone suppression. Int J Radiat Oncol Biol Phys 2012;82:1222-6. [Crossref] [PubMed]
- Pickles T, Graham P. Members of the British Columbia Cancer Agency Prostate Cohort Outcomes Initiative. What happens to testosterone after prostate radiation monotherapy and does it matter? J Urol 2002;167:2448-52. [Crossref] [PubMed]
- Daniell HW, Clark JC, Pereira SE, et al. Hypogonadism following prostate-bed radiation therapy for prostate carcinoma. Cancer 2001;91:1889-95. [Crossref] [PubMed]
- Underwood T, Giantsoudi D, Moteabbed M, et al. Can We Advance Proton Therapy for Prostate? Considering Alternative Beam Angles and Relative Biological Effectiveness Variations When Comparing Against Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys 2016;95:454-64. [Crossref] [PubMed]
- Guan F, Bronk L, Titt U, et al. Spatial mapping of the biologic effectiveness of scanned particle beams: towards biologically optimized particle therapy. Sci Rep 2015;5:9850. [Crossref] [PubMed]
- Paganetti H, Niemierko A, Ancukiewicz M, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys 2002;53:407-21. [Crossref] [PubMed]
- Mendenhall NP, Hoppe BS, Nichols RC, et al. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2014;88:596-602. [Crossref] [PubMed]
- Slater JD, Rossi CJ Jr, Yonemoto LT, et al. Proton therapy for prostate cancer: the initial Loma Linda University experience. Int J Radiat Oncol Biol Phys 2004;59:348-52. [Crossref] [PubMed]
- Nihei K, Ogino T, Onozawa M, et al. Multi-institutional Phase II study of proton beam therapy for organ-confined prostate cancer focusing on the incidence of late rectal toxicities. Int J Radiat Oncol Biol Phys 2011;81:390-6. [Crossref] [PubMed]
- Kim YJ, Cho KH, Pyo HR, et al. A phase II study of hypofractionated proton therapy for prostate cancer. Acta Oncol 2013;52:477-85. [Crossref] [PubMed]
- Pugh TJ, Munsell MF, Choi S, et al. Quality of life and toxicity from passively scattered and spot-scanning proton beam therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2013;87:946-53. [Crossref] [PubMed]
- Bryant C, Smith TL, Henderson RH, et al. Five-Year Biochemical Results, Toxicity, and Patient-Reported Quality of Life After Delivery of Dose-Escalated Image Guided Proton Therapy for Prostate Cancer. Int J Radiat Oncol Biol Phys 2016;95:422-34. [Crossref] [PubMed]
- Spratt DE, Pei X, Yamada J, et al. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2013;85:686-92. [Crossref] [PubMed]
- Vora SA, Wong WW, Schild SE, et al. Outcome and toxicity for patients treated with intensity modulated radiation therapy for localized prostate cancer. J Urol 2013;190:521-6. [Crossref] [PubMed]
- Edelman S, Liauw SL, Rossi PJ, et al. High-dose radiotherapy with or without androgen deprivation therapy for intermediate-risk prostate cancer: cancer control and toxicity outcomes. Int J Radiat Oncol Biol Phys 2012;83:1473-9. [Crossref] [PubMed]
- Gray PJ, Paly JJ, Yeap BY, et al. Patient-reported outcomes after 3-dimensional conformal, intensity-modulated, or proton beam radiotherapy for localized prostate cancer. Cancer 2013;119:1729-35. [Crossref] [PubMed]
- Hoppe BS, Michalski JM, Mendenhall NP, et al. Comparative effectiveness study of patient-reported outcomes after proton therapy or intensity-modulated radiotherapy for prostate cancer. Cancer 2014;120:1076-82. [Crossref] [PubMed]
- Fang P, Mick R, Deville C, et al. A case-matched study of toxicity outcomes after proton therapy and intensity-modulated radiation therapy for prostate cancer. Cancer 2015;121:1118-27. [Crossref] [PubMed]
- Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 2012;307:1611-20. [Crossref] [PubMed]
- Yu JB, Soulos PR, Herrin J, et al. Proton versus intensity-modulated radiotherapy for prostate cancer: patterns of care and early toxicity. J Natl Cancer Inst 2013;105:25-32. [Crossref] [PubMed]
- Verma V, Mishra MV, Mehta MP. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer 2016;122:1483-501. [Crossref] [PubMed]
- Konski A, Speier W, Hanlon A, et al. Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J Clin Oncol 2007;25:3603-8. [Crossref] [PubMed]
- Nichols RC, McIntyre K, Gifford J, et al. Intermediate-Risk Prostate Cancer: A Medicare-Based Cost Comparison of Five Radiotherapy Regimens. 97th Annual Meeting of the American Radium Society. Kauai, USA, 2015.
- Institute for Clinical and Economic Review. Final Appraisal Document. Brachytherapy & Proton Beam Therapy for Treatment of Clinically-Localized, Low-Risk Prostate Cancer. Available online: http://www.teambest.com/news/ICER.pdf
- Lundkvist J, Ekman M, Ericsson SR, et al. Proton therapy of cancer: potential clinical advantages and cost-effectiveness. Acta Oncol 2005;44:850-61. [Crossref] [PubMed]
- Medical Physics Web. MEVION S250 achieves outstanding first-year clinical results. Available online: http://medicalphysicsweb.org/cws/article/newsfeed/59853
- Michalski JM, Bae K, Roach M, et al. Long-term toxicity following 3D conformal radiation therapy for prostate cancer from the RTOG 9406 phase I/II dose escalation study. Int J Radiat Oncol Biol Phys 2010;76:14-22. [Crossref] [PubMed]
- Colaco RJ, Hoppe BS, Flampouri S, et al. Rectal toxicity after proton therapy for prostate cancer: an analysis of outcomes of prospective studies conducted at the university of Florida Proton Therapy Institute. Int J Radiat Oncol Biol Phys 2015;91:172-81. [Crossref] [PubMed]


