Reduced acute toxicity and improved efficacy from intensity-modulated proton therapy (IMPT) for the management of head and neck cancer
Introduction
Proton radiation therapy has been used for more than 20 years to treat cancer that arises in anatomically challenging head and neck locations. However, most patients with head and neck cancer to date have been treated with photon external-beam radiation therapy (EBRT). Significant improvements have been achieved over time in the planning and delivery of photon therapy, which has evolved from using 2-dimensional plans to 3-dimensional plans and on from there to the highly conformal technique of intensity-modulated radiation therapy (IMRT). These improvements have led to better radiation dose distribution and improved outcomes. However, proton therapy is becoming increasingly common, largely because of its dosimetric advantages. Protons have a Bragg peak, a unique physical characteristic that allows charged particles to deposit a large fraction of their energy at the end of their path. This largely spares distal structures from being exposed to exit dose beyond the tumor target. The heavier mass of protons also causes smaller scattering angles, which lead to sharper lateral dose distributions.
At this time, two types of proton therapy are in use: passive scatter or active scanning (also known as pencil beam proton therapy). In passive scatter therapy, the proton beam is spread by using either single- or double-scattering foils. This technique has several drawbacks; patient-specific devices are needed, which can be both expensive and labor-intensive to produce, particularly as tumors change shape over time. Also, the interaction of protons with the scattering material and collimators can lead to increased neutron production, which is associated with a risk of secondary malignancy. Finally, the scattering foils can absorb some of the energy of the protons and reduce the depth of penetration of the Bragg peak. In contrast, in active scanning proton therapy, magnets are used to deflect and steer the beam, which allows the entire treatment volume to be “painted” with the proton beams (1). The depth of the Bragg peak is modified by adjusting the energy of the beam before it enters the nozzle. Intensity-modulated proton therapy (IMPT) plans can be generated either by single-field optimization or by multiple-field optimization (2). Single-field optimization involves individually optimizing the spot intensities for each beam, whereas in multiple-field optimization, all beam intensities are optimized simultaneously to achieve an optimal dose distribution to the target and organs at risk of harboring subclinical disease (3). IMPT further provides greater dose conformality and a smaller integral dose than passive scatter proton therapy does.
The ability of IMPT or pencil beam proton therapy to direct high doses of radiation to tumors while sparing surrounding healthy tumors is particularly important in the treatment of head and neck malignancies. EBRT can allow some degree of organ preservation while avoiding some of the morbidity of surgery. However, traditional EBRT is also associated with significant morbidity because of the close proximity of several critical organ structures, as described further in the next paragraph. IMPT using pencil beam proton therapy has the potential to deliver tumoricidal radiation doses to tumors while sparing surrounding tissues, which would be expected to reduce morbidity and mortality. Notably, the precision of IMPT also has a disadvantage in that its accuracy can be compromised by changes in patient anatomy. Yeh et al. (4) showed that adaptive planning (i.e., repeated treatment planning over the course of therapy) is required to maintain initial dose constraints in the treatment of head and neck tumors; Palmer et al. (5) found that the fourth week of treatment is the optimal time for off-line adaptive planning.
With regard to side effects, head and neck cancers are of diverse histology and arise at a variety of anatomic subsites, including the paranasal sinuses, salivary glands, oral cavity, pharynx, and larynx. Side effects from EBRT are specific to the anatomic location of the cancer. However, the most common side effects encountered are xerostomia, dysphagia requiring short-term or permanent gastrostomy (feeding tube dependence), necrosis of soft tissue and bone, neck fibrosis, hearing impairment, optic neuropathy, temporal lobe necrosis, and secondary malignancies (6). A systematic review of in silico treatment-planning comparison studies demonstrated that protons have a dosimetric advantage over photon EBRT in that they provide lower doses to normal tissues, which raises the possibility of increasing the dose to a tumor without increasing toxicity (7). In this review, we assess the current state of the literature with regard to the effectiveness of IMPT, its acute toxicity, patient-reported outcomes (PROs), and the need for gastrostomy among patients with head and neck cancer.
Early findings on effectiveness and acute toxicity by organ site
Previous treatment-planning comparisons indicate that IMPT has considerable dosimetric advantages over IMRT and has the potential to reduce treatment-related toxicity, with equivalent or superior effectiveness, compared with IMRT. IMPT, however, is a relatively new technology, and published reports, albeit encouraging, are somewhat sparse. Two examples are studies published by Bhattasali et al. and Holliday et al., who used IMPT to treat patients with adenoid cystic carcinoma. At median follow-up times of 24.9 and 27 months, the local control rates in these studies were impressive at 88.9% and 93.8%, and toxicity was considered acceptable (8,9). These findings are a vast improvement over historical local control rates of 0% to 43% for traditional radiation therapy (8), although the possibility of selection bias cannot be ruled out. In the following sections, we discuss the current state of knowledge of the efficacy and toxicity of IMPT for head and neck cancer at different anatomic subsites. A summary of these results can be found in Table 1.
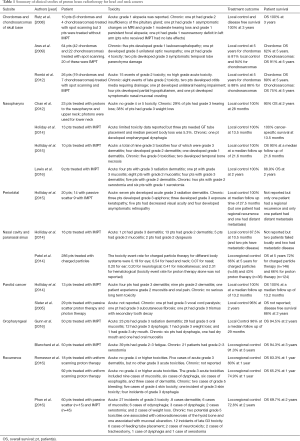
Full table
Chordomas and chondrosarcoma of the skull base
Both chordomas and chondrosarcomas of the skull base are radioresistant tumors. Early attempts at treatment with photon EBRT led to disappointingly low 5-year progression-free survival rates of <25% (10,11) and significant risks of serious toxicity (6). Combined proton and photon therapy, however, produced more encouraging results. In one such study reported by Munzenrider et al. (12), the 5-year local recurrence-free survival rate was 73% for patients with chordoma and 98% for patients with chondrosarcomas; another study from Noël et al. (13) revealed a local control rate of 54% at 4 years. Unfortunately, the toxicity of these treatments was unacceptably high: in the Munzenrider study, three patients died from brain stem injuries, eight had temporal lobe injuries, and other patients had hearing loss, cranial neuropathy and endocrinopathy (12). Another group used uniform scanning proton therapy for recurrent chordoma (14) and achieved much higher local control and overall survival (OS) rates (85% and 80% at 2 years). Unfortunately, re-irradiation was associated with high-grade acute and chronic toxicity, with one patient experiencing grade 3 laryngeal edema that necessitated a permanent tracheostomy and another grade 4 acute fourth ventricular obstruction that required placement of a shunt. Another patient experienced grade 3 chronic toxicity (radiation necrosis) and two others had grade 4 chronic toxicity (ischemic brainstem stroke and cerebrospinal fluid leak with meningitis).
The next generation of radiation therapy, IMPT, has had excellent efficacy with less toxicity relative to combined proton and photon EBRT. Rutz was the first to report the use of IMPT for skull-base chordoma and chondrosarcomas (15). In that small study, 10 patients were treated with spot scanning proton therapy (3 with IMPT) to a total dose of 66–74 Gy(RBE) without chemotherapy. The local control rate, disease-free survival rate, and OS rate were all 100% at 3 years, and no patient experienced acute toxicity that was worse than grade 1. However, 30% of these patients had some form of late toxicity. These early results were subsequently confirmed by Ares et al. (16) and Rombi et al. (17). Ares and colleagues reported results from 64 patients with skull-base chordoma or chondrosarcoma who had been treated to a mean dose of 68.4 Gy(RBE) with spot scanning proton beam radiation therapy. Twenty of these patients were treated with IMPT. At a mean follow-up time of 38 months, the estimated 5-year local control rate was 81% for chordomas and 94% for chondrosarcomas, and the estimated 5-year disease-specific survival rate was 81% for chordomas and 100% for chondrosarcomas. Toxicity results were also encouraging: at 5 years, 94% of patients were free from high-grade toxicity (two patients had grade 3–4 optic neuropathy and two had grade 3 symptomatic temporal lobe damage). The study reported by Rombi et al. involved 26 patients with chordoma or chondrosarcoma who were treated with spot scanning proton therapy; again, a subset of those patients received IMPT. Local control rates at 5 years were encouraging at 88% for chordomas and 86% for chondrosarcomas, as were 5-year survival rates (89% for chordomas and 75% for chondrosarcomas). No patient experienced acute or chronic toxicity that was worse than grade 2. These results demonstrate the potential of IMPT to improve local control and reduce toxicity relative to photon external-beam or combined proton and photon therapy.
Nasopharyngeal cancer
Nasopharyngeal carcinoma is common in Asia, and radiation therapy can lead to significant toxicity. The four most common types of severe (grade ≥3) toxicity after 2D radiotherapy as reported by Sanguineti et al. (18) are pituitary dysfunction, cranial nerve dysfunction, fibrosis and trismus. Late toxicity associated with 2D and conformal radiotherapy has also been reported in larger studies by Tuan et al. and Lee et al. (19,20), who highlighted the extremely narrow therapeutic margin for this type of cancer. IMRT with chemotherapy is often used for nasopharyngeal cancer, but combined proton and photon therapy has had promising results as well (21). Chan et al. published preliminary results of a phase II trial of 23 patients with stage III/IVb nasopharyngeal cancer. At a follow-up interval of 28 months, 2-year rates of local control, disease-free survival, and OS were 100%, 90%, and 100%. Side effects, however, were significant. Although no patient had grade 3 or higher xerostomia, 48% had a gastrostomy tube placed during treatment, 38% had grade 3 weight loss, and 29% had hearing loss (22).
To date, most of the published reports on IMPT for nasopharyngeal cancer have come from The University of Texas MD Anderson Cancer Center; initial results have been promising. In one of those studies, Lewis et al. compared the dose to 29 organs at risk on treatment plans for IMPT versus IMRT; mean doses to 15 of those organs were significantly different for the two modalities, with doses to 13 of those organs being lower on the IMPT plans (23). Another study (24) reported early outcomes for 13 patients given IMPT for nasopharyngeal cancer from 2011 to 2013; 2 of those patients had recurrent disease and had previously received photon RT, and the other 11 had newly diagnosed disease (five T1, two T2, two T3, and two T4; one N0, three N1, seven N2). Nine patients received induction chemotherapy and all patients received concurrent chemotherapy. During the median follow-up interval of 4.6 months (range, 1.8–9 months), side effects were mild, with a median percent body weight loss of 5.3% (range, 1.1% to 14%), with only one patient experiencing swallowing dysfunction. In another study from MD Anderson, 10 patients who received IMPT for nasopharyngeal cancer were case-matched with another 20 patients who received IMRT for nasopharyngeal cancer, with match criteria being T status, N status, radiation dose, chemotherapy type, WHO classification, sex, and age. Toxicity associated with IMPT, quantified in terms of gastrostomy tube placement, was significantly lower among patients treated with IMPT (20% vs. 65% for IMRT, P=0.02). Notably, no patient who received a mean oral cavity dose of <26 Gy required a gastrostomy tube (25). Future prospective clinical trials are planned with the goals of reducing the toxicity associated with treatment of nasopharyngeal cancer as well as improving disease outcomes for patients with T4 disease.
Periorbital tumors
Periorbital tumors are quite rare, with an estimated incidence of only 0.072 per 100,000 people (24). This low prevalence precludes using randomized controlled trials to determine the optimal treatment for periorbital tumors. To date, most cases have been managed with orbital exenteration, with adjuvant radiation therapy or chemoradiation depending on the pathologic findings. Some evidence exists to suggest that postoperative radiation can improve clinical outcomes; photon EBRT has been shown in retrospective analyses to improve local control rates by up to 50% (24). However, radiation is also associated with acute toxicity, most often conjunctivitis, loss of eyelashes, xerophthalmia, and punctate erosion of the cornea (26). Proton therapy may be able to reduce the toxicity burden to ocular structures after orbit-sparing surgery, which would complete the “trifecta” of cure, cosmesis, and function. In a landmark study by Holliday et al. (27), 20 patients who had had orbit-sparing surgery for a new diagnosis of periorbital cancer received postoperative IMPT (n=6) or passive scatter proton therapy (n=14). The median radiation dose to the tumor was 60 Gy(RBE) [range, 50–70 Gy(RBE)]; 11 patients had concurrent chemotherapy; and the median follow-up time was 27.1 months (range, 2.6–77.2 months). No patient experienced grade 4 or grade 5 toxicity; three patients developed chronic grade 3 epiphora and three developed grade 3 exposure keratopathy. Visual acuity dropped below baseline levels in four patients, but all four maintained vision sufficient to perform all activities of daily living without difficulty. The maximum dose to the ipsilateral cornea was related to grade 3 chronic ocular toxicity: no patient whose maximum dose to the ipsilateral cornea of <36 Gy(RBE) developed grade 3 chronic toxicity, but 46% of those who received >36 Gy(RBE) developed grade 3 chronic toxicity. Also notable was the finding that grade 3 acute toxicity seemed to depend on treatment modality, with 67% of the IMPT group having grade 3 acute toxicity versus 21% of the passive scatter group (P=0.052). At this time, a multidisciplinary approach to periorbital tumors that includes proton therapy seems essential for ensuring the highest cure rates with excellent cosmesis and preservation of vision.
Nasal cavity and paranasal sinus cancers
Nasal cavity and paranasal sinus tumors are typically treated with surgery followed by postoperative EBRT. This therapy can produce good 5-year local control rates (82% for T1–3 tumors and 50% for T4 tumors) (28), but it can also cause blindness in up to 38% of patients owing to retinopathy and optic neuropathy (29-31). Combined proton and photon therapy and passive scatter proton therapy seem to have better toxicity and tumor control. Fitzek et al. (32) and Resto et al. (33) both described their experience with combined proton and photon therapy. In Fitzek’s study of 19 patients with olfactory neuroblastoma, the 5-year local control rate was 88% and no patients experienced radiation-induced vision loss, although four patients had evidence of radiation-induced damage on magnetic resonance imaging. The larger study reported by Resto et al. included 102 patients with locally advanced sinonasal cancer; 5-year local control rates were 95% for patients who had complete resection, 82% for those with a partial resection, and 87% for those who had biopsy only. A prospective non-randomized trial reported by Okano et al. (34) involved using passive scatter proton therapy to treat 13 patients with T4b nasal and sinonasal cancers; results of that study were excellent, with 85% of patients achieving complete response and no patients experiencing blindness or acute brain damage. These positive results were corroborated by a meta-analysis by Patel et al. (35) comparing outcomes after charged particle therapy versus photon therapy. In that study, charged particle therapy led to superior 5-year rates of OS, disease-free survival and local-regional control over IMRT.
Given the relative rarity of nasal cavity and paranasal sinus cancers, little is known of the potential advantage of IMPT for these tumors, and randomized controlled trials are unlikely. At MD Anderson, Holliday and Frank described initial results for 16 patients with nasal or paranasal sinus tumors treated with IMPT (24), 6 with adenoid cystic carcinoma, 6 neuroblastoma, 3 squamous cell carcinoma, and 1 with an undifferentiated carcinoma. The median follow-up time was 10.2 months (range, 1.33–20.3 months). Thirteen patients had received IMPT after resection and 3 received IMPT as definitive treatment; disease was newly diagnosed in 12 patients and recurrent in the other 4. The median radiation dose received was 62 Gy(RBE) [range, 60–70 Gy(RBE)]; 11 patients received concurrent chemotherapy and 2 received induction chemotherapy. Treatment was well tolerated, with no grade 4 or 5 toxicity. One patient had grade 3 dermatitis; 13 had grade 2 dermatitis; 5 had grade 2 mucositis; and 2 had grade 2 dysgeusia. Eight patients required opioids for pain management during treatment, and 2 patients required a gastrostomy tube for nutritional support. At last follow-up, no patient had grade 3–5 late effects. Of the 13 patients who had surgery before IMPT, 10 had no evidence of disease, 2 had metastatic disease, and 1 had locally recurrent disease. Of the three patients who received IMPT with definitive intent, two had stable disease and one had progressive disease.
Parotid tumors
Salivary tumors are also fairly uncommon, contributing less than 5% of all head and neck tumors (36). Surgery is the main form of treatment for parotid tumors, but certain subtypes of tumors and tumors with certain high-risk features are treated with postoperative radiation. Local control and OS rates for patients given postoperative radiation therapy are 73–94% and 69–78% (37,38). Little has been published on proton therapy for parotid tumors. One dosimetric study comparing proton therapy with IMRT showed that proton therapy was associated with significantly lower doses to the contralateral parotid gland and submandibular gland, spinal cord, brainstem, and oral cavity (39). Another study reported by Romesser et al. (40) compared acute toxicity between uniform scanning proton therapy and IMRT for patients with recurrence or metastatic disease to the major salivary glands. That study showed that grade ≥2 acute effects such as dysgeusia, mucositis, and nausea were less common among patients treated with protons. The only other published study to date has been from MD Anderson (24), reporting 13 patients with parotid tumors (4 with metastatic disease and 9 with newly diagnosed disease) who received IMPT from 2011 to 2013. Twelve of these patients had received surgery before radiation; 1 patient had had induction chemotherapy (for borderline resectable disease) followed by chemoradiation and surgery. Radiation doses ranged from 50 to 70 Gy(RBE) [median 60 Gy(RBE)]. At a median follow-up time of 13.2 months (range, 3.2–15.9 months), all patients had completed radiation as planned; treatment was well tolerated, with no grade 4 or grade 5 toxicity. Four patients had grade 3 dermatitis, nine patients had grade 2 dermatitis, and one patient had grade 2 mucositis and oral pain. Two patients required opioids for pain management. The median change in body weight was −1.3% and ranged from −3.7% to +3.1%. No patient required a gastrostomy tube, and no patient had any serious late toxicity by the time of last follow-up. When that review was published, none of the patients had any evidence of recurrent or metastatic disease.
Oropharyngeal cancer
Radiation therapy, with or without chemotherapy, is typically used to treat oropharyngeal cancer, but local failure and acute toxicity are common with this treatment. Common acute effects include mucositis, dermatitis, dysphagia, sore throat, odynophagia, thickened secretions, in-field alopecia, xerostomia, and taste disturbance (41). Historically, local control rates have been suboptimal, being only 55% in the concomitant boost arm of the RTOG 9003 trial (42). Slater et al. (43) described 29 patients with stage II–IV oropharyngeal cancer treated with a combination of passive scatter proton therapy and photon therapy to a total dose of 75.9 Gy(RBE), with promising results. Although the follow-up period varied from 2 to 96 months, the actuarial 2-year local control rate was 96%. Only three incidents of grade 3 late toxicity were reported, namely a vocal cord paralysis, subcutaneous fibrosis, and trismus with secondary tooth decay. Acute toxicity rates were not reported, but all patients were said to have been able to complete treatment as planned, with the aid of nutritional and anesthetic support.
In a recent case-matched control analysis of IMRT and IMPT plans, the IMPT plans delivered significantly lower doses to the anterior and posterior oral cavity, hard palate, larynx, mandible, esophagus, and several central nervous system structures involved in the nausea and vomiting response (44). This improved dose distribution may lead to a decrease in treatment-related toxicity. Gunn and colleagues from MD Anderson recently published results from 50 patients with oropharyngeal cancer treated with IMPT (45). In that study, overall and progression-free survival rates at 2 years were 94.5% and 88.6%. Toxicity was considered encouraging, with no patients having grade 4 or 5 acute or late toxicity. Grade 3 acute toxicity was relatively common and included 23 incidents of radiation dermatitis, 29 oral mucositis, 12 dysphagia, 1 weight loss, and 1 dry mouth; grade 3 late toxicity consisted of 6 incidents of dysphagia, 1 dry mouth, and 1 oral mucositis. The median body weight loss was 7.4%, and 12 patients required gastrostomy tube placement. Blanchard and colleagues used a case-matched design to compare 50 patients with oropharyngeal cancer who received IMPT with 100 patients with the same type of cancer treated with IMRT (46). Rates of severe weight loss and feeding tube placement were both lower for patients who received IMPT, and OS and progression-free survival rates at 3 years for the IMPT group were 94.3% and 85.8%. In terms of toxicity, 12 patients in the IMPT group had gastrostomy tubes placed, and 39 had grade 2–3 fatigue during treatment. At 3 months after treatment, 4 patients had lost more than 20% of their baseline body weight and 21 had grade 2–3 xerostomia. Currently, a multi-institutional randomized controlled phase II/III trial is underway to compare the incidence and severity of chronic grade 3–5 toxicity for patients with advanced oropharyngeal cancer treated with IMPT or IMRT [NCT01893307, “Intensity-Modulated Proton Beam Therapy (IMPT) Versus Intensity-Modulated Photon Therapy (IMRT) for the Treatment of Oropharyngeal Cancer of the Head and Neck”]. The results of this trial are expected to help clarify the extent to which IMPT can reduce toxicity for patients with oropharyngeal cancer.
Recurrent head and neck cancer
A significant proportion of patients with head and neck cancer will need repeated radiation therapy for disease that recurs after definitive treatment; indeed, local-regional recurrence is the leading cause of death for such patients (47). Re-irradiation can be curative but also has significant toxic side effects, with rates of grade ≥3 toxicity ranging from 34% to 65% (48). These forms of toxicity can include trismus, osteoradionecrosis, subcutaneous fibrosis, late mucosal side effects, pharyngeal, laryngeal, esophageal dysfunction, or carotid ruptures (48). Survival rates after conventional re-irradiation are less than optimal, ranging from 26% to 57% at 2 years (49). Some preliminary evidence exists to suggest that proton therapy may reduce toxicity and improve survival among patients needing re-irradiation. For instance, Stuschke et al. found that IMPT was better able to spare normal tissues than was helical tomotherapy (48). Romesser et al. (40) analyzed patients with recurrent head and neck cancer, comparing 18 patients who received uniform scanning proton therapy with 23 patients who received IMRT. That study showed that patients treated with protons had significantly lower rates of grade ≥2 acute dysgeusia, mucositis, and nausea. Actuarial rates of local-regional control and OS for the proton group at 1 year were 80% and 83.3%. No patient in the proton group had grade ≥4 toxicity; five had acute grade 3 dermatitis, but no other forms of grade 3 acute toxicity were experienced. A more recent multi-institutional study by the same group (50) analyzed 92 patients treated with uniform scanning proton therapy for recurrent head and neck cancer; rates of local-regional control and OS at 1 year were 74.9% and 65.2%. No patients experienced grade ≥4 acute toxicity; as for grade 3 acute toxicity, nine patients had mucositis, six had dysphagia, six had esophagitis, and three had dermatitis. Late effects included two cases of grade 5 bleeding and five of grade 4 skin toxicity; one patient had late grade 3 skin toxicity and four had late grade 3 dysphagia. Finally, 32 patients (35%) received a gastrostomy tube during this study. These encouraging results were recently confirmed by Phan et al. (49), who examined 60 patients treated with either passive scatter proton therapy (n=15) or IMPT (n=45); rates of local-regional control and OS at 2 years were 72.8% and 69.7%. The 27 incidents of grade 3 toxicity consisted of 8 cases of dermatitis, 6 mucositis, 6 odynophagia, 3 dysphagia, 2 xerostomia, and 2 weight loss. Thirteen of the patients in that study (22%) required placement of a gastrostomy tube. As for late effects, two patients developed possibly treatment-related late grade 5 toxicity, one with osteoradionecrosis of the hyoid bone and another with mucosal ulceration. The 12 incidents of late grade 3 toxicity consisted of 6 patients requiring feeding tubes, 2 with neurotoxicity, 2 with tracheostomy, 1 with dysphagia, and 1 with xerostomia. In summary, these early results suggest that re-irradiation with protons seems to offer favorable survival, tumor control, and toxicity relative to conventional radiation therapy. However, more patients, and large prospective trials, are needed to confirm these results.
PROs associated with IMPT
PRO surveys are increasingly being used to assess symptom burden and quality of life from the patient’s perspective, without the need to rely on a third party (51). PROs may also reflect symptom burden more accurately, as patient-reported symptoms are often more severe than those assessed by physicians (52). Investigators at MD Anderson recently completed the first study of PROs among patients given IMPT for oropharyngeal tumors (53). That study involved giving 35 patients who had received IMPT and 46 patients who had received IMRT the MD Anderson Symptom Inventory-Head and Neck Module (MDASI-HN), a validated tool that has been shown to correlate well with some physician-assessed outcomes such as mucositis (54). Patients were given questionnaires to document their symptoms at baseline, during treatment, during the subacute recovery phase (from the end of treatment to 3 months after treatment), and >3 months after treatment. No statistically significant differences were found between groups in symptom burdens at baseline, during treatment, or >3 months after treatment; however, the patients who received IMPT had a lower symptom burden during the subacute recovery phase than did the IMRT patients. This suggests that symptoms for patients given IMPT may return to baseline more quickly than for patients who received IMRT. The apparent improvements in dose distribution for IMPT relative to photon therapy might be expected to decrease the symptom burden for patients with head and neck cancer. Trial NCT01893307, the comparison of IMPT and IMRT for oropharyngeal cancer, also includes several PRO instruments that will provide additional prospective data on IMPT and symptom burden among patients receiving concurrent chemoradiation for advanced oropharyngeal tumors.
Gastrostomy tube and nutritional requirements during and after IMPT
A gastrostomy tube is often necessary to maintain appropriate hydration and nutrition status during radiation treatment for head and neck cancer; however, tube placement is an invasive procedure with reported minor complication rates of 19.5%, major complication rates of 7.45%, and mortality rates of 2.2% (55). Tube placement is also expensive, with one estimate indicating $31,832 for placement and use of a gastrostomy tube for 1 year (56). Many symptoms can prompt placement of a gastrostomy tube, including mucositis, xerostomia, dysphagia, dysgeusia, and nausea (25). The dosimetric advantages of IMPT have the potential to reduce the dose to organs at risk and thereby decrease the related symptoms and possibly reduce the number of tubes needed. A case-matched control study reported by Holliday and others (25) of patients with nasopharyngeal carcinoma who received IMRT or IMPT showed that IMPT outperformed IMRT with respect to the need for gastrostomy tubes (20% in IMPT vs. 65% in IMRT, P=0.02) and dose to several organs at risk. On multivariate analysis, higher mean dose to the oral cavity was significantly associated with higher tube-placement rates, and IMPT was associated with a lower oral cavity dose than IMRT. Specifically, no patient who received <26 Gy(RBE) to the oral cavity dose had a gastrostomy tube placed, but all patients who received >41.8 Gy required a tube. IMPT was also associated with significantly lower mean doses to the brain stem, whole brain, and mandible. Also, as noted previously, Blanchard and colleagues compared feeding-tube placement rates and body weight loss among 50 patients with oropharyngeal cancer treated with IMPT and 100 patients with oropharyngeal cancer treated with IMRT (46). IMPT was associated with reduced rates of feeding tube placement and severe weight loss both during and after treatment. During treatment, only 24% of IMPT patients had a gastrostomy tube placed compared with 38% of IMRT patients. By 3 months after treatment, 18% of IMPT patients had lost >20% of their baseline body weight or had a gastrostomy tube, versus 34% of IMRT patients (P=0.05). At 1 year after treatment, these percentages had dropped to 8% for the IMPT group and 27% for the IMRT group (P=0.008). As noted previously, the ongoing clinical trial NCT01893307 is underway to compare the incidence and severity of toxicity associated with IMRT and IMPT. Enrollment in this study, and others like it, is necessary to establish whether IMPT can consistently reduce toxicity and reduce the need for gastrostomy tubes.
Conclusions
Early trials of IMPT have shown promising results, suggesting that proton therapy is safe and effective for the management of head and neck tumors. IMPT eliminates the unnecessary radiation delivered to proximal organs and structures associated with IMRT and seems to be associated with improved PROs during the first 3 months after treatment, decreased gastrostomy tube dependence, and lower rates of grade ≥3 toxicity while resulting in equivalent or improved effectiveness for tumors at a variety of anatomical subsites of the head and neck. Ongoing and future prospective clinical trials will further establish the degree to which IMPT can enhance tumor control and reduce symptom burden.
Acknowledgements
Funding: Funded in part by Cancer Center Support (Core) Grant CA0176672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Smith A, Gillin M, Bues M, et al. The M. D. Anderson proton therapy system. Med Phys 2009;36:4068-83. [Crossref] [PubMed]
- Zhu XR, Poenisch F, Li H, et al. A single-field integrated boost treatment planning technique for spot scanning proton therapy. Radiat Oncol 2014;9:202. [Crossref] [PubMed]
- Liu W, Zhang X, Li Y, et al. Robust optimization of intensity modulated proton therapy. Med Phys 2012;39:1079-91. [Crossref] [PubMed]
- Yeh BK, Georges RH, Zhu XR, et al. Adaptive replanning is required during intensity modulated proton therapy for head-and-neck cancers. Int J Radiat Oncol Biol Phys 2012;84:S56-7. [Crossref]
- Palmer M, Jones T, Waddell M, et al. The optimal timing for off-line adaptive planning for bilateral head-and-neck IMPT is week 4. Int J Radiat Oncol Biol Phys 2012;84:S479-80. [Crossref]
- Holliday EB, Frank SJ. Proton radiation therapy for head and neck cancer: a review of the clinical experience to date. Int J Radiat Oncol Biol Phys 2014;89:292-302. [Crossref] [PubMed]
- van de Water TA, Bijl HP, Schilstra C, et al. The potential benefit of radiotherapy with protons in head and neck cancer with respect to normal tissue sparing: a systematic review of literature. Oncologist 2011;16:366-77. [Crossref] [PubMed]
- Bhattasali O, Holliday E, Kies MS, et al. Definitive proton radiation therapy and concurrent cisplatin for unresectable head and neck adenoid cystic carcinoma: A series of 9 cases and a critical review of the literature. Head Neck 2016;38 Suppl 1:E1472-80. [Crossref] [PubMed]
- Holliday E, Bhattasali O, Kies MS, et al. Postoperative intensity-modulated proton therapy for head and neck adenoid cystic carcinoma. Int J Particle Ther 2016;2:533-43. [Crossref]
- Catton C, O'Sullivan B, Bell R, et al. Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol 1996;41:67-72. [Crossref] [PubMed]
- Zorlu F, Gürkaynak M, Yildiz F, et al. Conventional external radiotherapy in the management of clivus chordomas with overt residual disease. Neurol Sci 2000;21:203-7. [Crossref] [PubMed]
- Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol 1999;175 Suppl 2:57-63. [Crossref] [PubMed]
- Noël G, Feuvret L, Calugaru V, et al. Chordomas of the base of the skull and upper cervical spine. One hundred patients irradiated by a 3D conformal technique combining photon and proton beams. Acta Oncol 2005;44:700-8. [Crossref] [PubMed]
- McDonald MW, Linton OR, Shah MV. Proton therapy for reirradiation of progressive or recurrent chordoma. Int J Radiat Oncol Biol Phys 2013;87:1107-14. [Crossref] [PubMed]
- Rutz HP, Weber DC, Goitein G, et al. Postoperative spot-scanning proton radiation therapy for chordoma and chondrosarcoma in children and adolescents: initial experience at Paul Scherrer Institute. Int J Radiat Oncol Biol Phys 2008;71:220-5. [Crossref] [PubMed]
- Ares C, Hug EB, Lomax AJ, et al. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys 2009;75:1111-8. [Crossref] [PubMed]
- Rombi B, Ares C, Hug EB, et al. Spot-scanning proton radiation therapy for pediatric chordoma and chondrosarcoma: clinical outcome of 26 patients treated at Paul Scherrer Institute. Int J Radiat Oncol Biol Phys 2013;86:578-84. [Crossref] [PubMed]
- Sanguineti G, Geara FB, Garden AS, et al. Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of local and regional control. Int J Radiat Oncol Biol Phys 1997;37:985-96. [Crossref] [PubMed]
- Tuan JK, Ha TC, Ong WS, et al. Late toxicities after conventional radiation therapy alone for nasopharyngeal carcinoma. Radiother Oncol 2012;104:305-11. [Crossref] [PubMed]
- Lee AW, Ng WT, Hung WM, et al. Major late toxicities after conformal radiotherapy for nasopharyngeal carcinoma-patient- and treatment-related risk factors. Int J Radiat Oncol Biol Phys 2009;73:1121-8. [Crossref] [PubMed]
- Holliday EB, Frank SJ. Proton therapy for nasopharyngeal carcinoma. Chin Clin Oncol 2016;5(4):25. [Crossref] [PubMed]
- Chan A, Adams JA, Weyman E, et al. A phase II trial of proton radiation therapy with chemotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2012;84:S151-2. [Crossref]
- Lewis GD, Holliday EB, Kocak-Uzel E, et al. Intensity-modulated proton therapy for nasopharyngeal carcinoma: Decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck 2016;38 Suppl 1:E1886-95. [Crossref] [PubMed]
- Holliday EB, Frank SJ. Current applications of proton beam radiation for the treatment of head and neck tumors. Otorinolaringol 2014;64:1-11.
- Holliday EB, Garden AS, Rosenthal DI, et al. Proton therapy reduces treatment-related toxicities for patients with nasopharyngeal cancer: a case-match control study of intensity modulated proton therapy (IMPT) and intensity modulated photon therapy (IMRT). Int J Particle Ther 2015;2:19-28. [Crossref]
- Jeganathan VS, Wirth A, MacManus MP. Ocular risks from orbital and periorbital radiation therapy: a critical review. Int J Radiat Oncol Biol Phys 2011;79:650-9. [Crossref] [PubMed]
- Holliday EB, Esmaeli B, Pinckard J, et al. A multidisciplinary orbit-sparing treatment approach that includes proton therapy for epithelial tumors of the orbit and ocular adnexa. Int J Radiat Oncol Biol Phys 2016;95:344-52. [Crossref] [PubMed]
- Mendenhall WM, Amdur RJ, Morris CG, et al. Carcinoma of the nasal cavity and paranasal sinuses. Laryngoscope 2009;119:899-906. [Crossref] [PubMed]
- Waldron JN, O'Sullivan B, Warde P, et al. Ethmoid sinus cancer: twenty-nine cases managed with primary radiation therapy. Int J Radiat Oncol Biol Phys 1998;41:361-9. [Crossref] [PubMed]
- Takeda A, Shigematsu N, Suzuki S, et al. Late retinal complications of radiation therapy for nasal and paranasal malignancies: relationship between irradiated-dose area and severity. Int J Radiat Oncol Biol Phys 1999;44:599-605. [Crossref] [PubMed]
- Katz TS, Mendenhall WM, Morris CG, et al. Malignant tumors of the nasal cavity and paranasal sinuses. Head Neck 2002;24:821-9. [Crossref] [PubMed]
- Fitzek MM, Thornton AF, Varvares M, et al. Neuroendocrine tumors of the sinonasal tract. Results of a prospective study incorporating chemotherapy, surgery, and combined proton-photon radiotherapy. Cancer 2002;94:2623-34. [Crossref] [PubMed]
- Resto VA, Chan AW, Deschler DG, et al. Extent of surgery in the management of locally advanced sinonasal malignancies. Head Neck 2008;30:222-9. [Crossref] [PubMed]
- Okano S, Tahara M, Zenda S, et al. Induction chemotherapy with docetaxel, cisplatin and S-1 followed by proton beam therapy concurrent with cisplatin in patients with T4b nasal and sinonasal malignancies. Jpn J Clin Oncol 2012;42:691-6. [Crossref] [PubMed]
- Patel SH, Wang Z, Wong WW, et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol 2014;15:1027-38. [Crossref] [PubMed]
- Rodriguez CP, Parvathaneni U, Méndez E, et al. Salivary gland malignancies. Hematol Oncol Clin North Am 2015;29:1145-57. [Crossref] [PubMed]
- Armstrong JG, Harrison LB, Spiro RH, et al. Malignant tumors of major salivary gland origin. A matched-pair analysis of the role of combined surgery and postoperative radiotherapy. Arch Otolaryngol Head Neck Surg 1990;116:290-3. [Crossref] [PubMed]
- Garden AS, el-Naggar AK, Morrison WH, et al. Postoperative radiotherapy for malignant tumors of the parotid gland. Int J Radiat Oncol Biol Phys 1997;37:79-85. [Crossref] [PubMed]
- Kandula S, Zhu X, Garden AS, et al. Spot-scanning beam proton therapy vs intensity-modulated radiation therapy for ipsilateral head and neck malignancies: a treatment planning comparison. Med Dosim 2013;38:390-4. [Crossref] [PubMed]
- Romesser PB, Cahlon O, Scher E, et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol 2016;118:286-92. [Crossref] [PubMed]
- Cannon GM, Adelstein DJ, Gentry LR, et al. Oropharyngeal cancer. In: Bogart JA, Buchholz TA, Foote RL. editors. Clinical Radiation Oncology. 4th Edition. Philadelphia: Elsevier, 2016:597-628.e6.
- Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys 2000;48:7-16. [Crossref] [PubMed]
- Slater JD, Yonemoto LT, Mantik DW, et al. Proton radiation for treatment of cancer of the oropharynx: early experience at Loma Linda University Medical Center using a concomitant boost technique. Int J Radiat Oncol Biol Phys 2005;62:494-500. [Crossref] [PubMed]
- Holliday EB, Kocak-Uzel E, Feng L, et al. Dosimetric advantages of intensity-modulated proton therapy for oropharyngeal cancer compared with intensity-modulated radiation: A case-matched control analysis. Med Dosim 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Gunn GB, Blanchard P, Garden AS, et al. Clinical outcomes and patterns of disease recurrence after intensity modulated proton therapy for oropharyngeal squamous carcinoma. Int J Radiat Oncol Biol Phys 2016;95:360-7. [Crossref] [PubMed]
- Blanchard P, Garden AS, Gunn GB, et al. Intensity-modulated proton beam therapy versus intensity-modulated photon therapy for patients with oropharyngeal cancer: A case-matched analysis. Radiother Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Biagioli MC, Harvey M, Roman E, et al. Intensity-modulated radiotherapy with concurrent chemotherapy for previously irradiated, recurrent head and neck cancer. Int J Radiat Oncol Biol Phys 2007;69:1067-73. [Crossref] [PubMed]
- Stuschke M, Kaiser A, Abu-Jawad J, et al. Re-irradiation of recurrent head and neck carcinomas: comparison of robust intensity modulated proton therapy treatment plans with helical tomotherapy. Radiat Oncol 2013;8:93. [Crossref] [PubMed]
- Phan J, Sio TT, Nguyen TP, et al. Reirradiation of head and neck cancers with proton therapy: outcomes and analyses. Int J Radiat Oncol Biol Phys 2016;96:30-41. [Crossref] [PubMed]
- Romesser PB, Cahlon O, Scher ED, et al. Proton Beam Reirradiation for recurrent head and neck cancer: Multi-institutional report on feasibility and early outcomes. Int J Radiat Oncol Biol Phys 2016;95:386-95. [Crossref] [PubMed]
- Turk DC, Dworkin RH, Burke LB, et al. Developing patient-reported outcome measures for pain clinical trials: IMMPACT recommendations. Pain 2006;125:208-15. [Crossref] [PubMed]
- Meirovitz A, Murdoch-Kinch CA, Schipper M, et al. Grading xerostomia by physicians or by patients after intensity-modulated radiotherapy of head-and-neck cancer. Int J Radiat Oncol Biol Phys 2006;66:445-53. [Crossref] [PubMed]
- Sio TT, Lin HK, Shi Q, et al. Intensity modulated proton therapy versus intensity modulated photon radiation therapy for oropharyngeal cancer: first comparative results of patient-reported outcomes. Int J Radiat Oncol Biol Phys 2016;95:1107-14. [Crossref] [PubMed]
- Rosenthal DI, Mendoza TR, Fuller CD, et al. Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: a prospective analysis using the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module. Cancer 2014;120:1975-84. [Crossref] [PubMed]
- Grant DG, Bradley PT, Pothier DD, et al. Complications following gastrostomy tube insertion in patients with head and neck cancer: a prospective multi-institution study, systematic review and meta-analysis. Clin Otolaryngol 2009;34:103-12. [Crossref] [PubMed]
- Callahan CM, Buchanan NN, Stump TE. Healthcare costs associated with percutaneous endoscopic gastrostomy among older adults in a defined community. J Am Geriatr Soc 2001;49:1525-9. [Crossref] [PubMed]


