Treatment of common pediatric CNS malignancies with proton therapy
Introduction
Primary CNS tumors represent a large proportion of cancers occurring in children and adolescents under the age of 19 years (yr). As per the Central Brain Tumor Registry of the United States (CBTRUS), there is an estimated average age-adjusted annual incidence of 5.42 per 100,000 for brain and CNS neoplasms (1). The World Health Organization (WHO) provides a comprehensive scheme by which childhood CNS tumors can be categorized and graded, in an effort to stratify tumors based on biological behaviors. Through advents in classification, and significant advancements in the treatment of childhood malignancies, the 5 yrs survival rates for childhood cancers have improved dramatically, with a 5 yrs survival rate of 74% for CNS childhood malignancies diagnosed between 2004–2010 (2). Radiation therapy plays an integral role in the management of childhood CNS tumors. However, with the rising number of long-term survivors of childhood malignancies who have received radiation therapy, late effects of therapy have become apparent. These late effects manifest in a multitude of presentations including impairment of neurocognitive development, hormonal dysfunction secondary to hypothalamic-pituitary axis radiation with resultant growth or gonadal dysfunction, as well increased risk of secondary malignancies (3). In an attempt to reduce unnecessary radiation dose to organs at risk for late effects, there has been a strong movement to utilize increasingly conformal modes of radiation therapy including three dimensional conformal radiation therapy (3D-CRT), intensity modulated radiation therapy (IMRT), volumetric modulated arc therapy (VMAT), as well as proton therapy (PRT). Compared to photon therapy, PRT has a region of high dose deposition, with minimal dose delivery distal to the region of the Bragg Peak. This is in stark contrast to photons, which enter with high energies and deposit their dose not only throughout the tumor but also distal to the target structure. As such, PRT allows significant reduction of the overall integral dose a child receives, potentially reducing risk of late side effects of such a dose “bath”. Our hope in this review is to highlight the current standards, and potential benefit of PRT in common childhood brain tumors.
Low grade gliomas
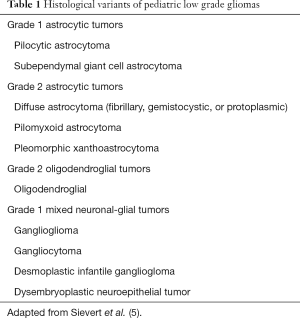
Gliomas represent a basket term for tumors arising from neuroepithelial constituents (i.e., astrocytes, oligodendrocytes, mixed neuronal-glial). Low grade gliomas (WHO grade I or II) comprise approximately 40% of primary CNS tumors of childhood, with the most common subtypes being WHO grade I pilocytic astrocytoma, and WHO grade II diffuse fibrillary astrocytoma (4). Other less common histologies of low-grade gliomas are noted in Table 1. These tumors frequently arise in the cerebellum, followed by hemispheric (cerebral), deep midline structures, optic pathway and brain stem (5). Location plays an integral role in determining the possibility of a complete resection, since a gross total resection (GTR) is the mainstay of therapy. Long term outcomes, with 5 yrs OS of 100% as well as 5 yrs PFS rates of 90%, are promising in the setting of a GTR and routine surveillance (6). Post-operative management in the setting of subtotal resection (STR) or biopsy is controversial. Various series report the potential benefit of immediate postoperative radiation therapy in reducing risk of progression, however, given the indolent nature of the disease many patients may not progress despite STR. As noted by Fisher et al. in a series evaluating the outcomes of children who underwent STR for low-grade gliomas, at a median follow up of 7.3 yrs, 58% of patients who did not receive postoperative RT were free from progression. While immediate postoperative radiation therapy after STR resulted in a lower rate of progression, there was no overall survival benefit compared to the deferred RT group (7). Radiation therapy after STR is generally delayed until tumor progression to allow for maximal milestone development. Immediate RT may be more strongly considered if progression could risk serious damage to critical sites.
Full table
Radiation therapy is also warranted for symptomatic disease (at diagnosis or recurrence) that is not amenable to surgical intervention in an effort to prevent additional symptoms, tumor progression and possible symptom relief.
In the interim, chemotherapy can be utilized to delay progression. A regimen of carboplatin/vincristine has shown promising control rates achieving 3 yr PFS of 68% in a series of children, including those <5 yrs of age, with newly diagnosed low grade gliomas that underwent minimal debulking of <50% of the tumor at time of surgery (8). Other forms of systemic therapy currently being evaluated in low grade gliomas stem from an understanding that BRAF mutations (i.e., V600E activating mutations, BRAF gene fusion) that result in activation of the mitogen-activated protein kinase (MAPK) pathway represent a common alteration in this cohort of tumors. As such, current protocols are assessing the role of targeted therapy such as MEK and BRAF inhibitors in the setting of recurrent or refractory low-grade gliomas. Similarly, tissue microarray analyses of low-grade glioma samples have revealed high expression of epidermal growth factor receptor (EGFR) and mechanistic target of rapamycin (mTOR), resulting in current trials that are evaluating disease control/response rates with mTOR and EGFR inhibitors for refractory/recurrent tumors (9,10).
Modern highly conformal radiotherapy techniques have been evaluated in the treatment of low-grade glioma. Merchant et al. evaluated disease control in a series of patients with sub-totally resected low-grade gliomas treated with primarily 3D-CRT to a dose of 54 Gy with the intention to conform CTV margins to 1cm of gross residual tumor and/or tumor bed. Their results revealed excellent local control rates with their specified margins with 5 and 10 yrs EFS of 87.4% and 74.3%, respectively. Ten yrs overall survival rates approached 96% (11). These favorable results prompted evaluation of the role of PRT in this population. Hug et al. evaluated a series of 27 pediatric patients with unresected or sub-totally resected low-grade astrocytomas located in the cerebral hemispheres, diencephalon or brainstem treated with fractionated 3D planned PRT in an effort to assess safety and efficacy of this therapeutic modality. Treatment volumes included the GTV, which was delineated as enhancement on CT or MR with a surrounding margin of 0.5–1 cm to designate the CTV. PRT was delivered in a fractionated manner of 1.8 cobalt gray equivalent (CGE) per fraction, one treatment per day, five days per week, with a prescription dose of 50.4 to 63 Gy CGE assuming a relative biological effectiveness (RBE) of 1.1. With a mean follow up of 39 months, the cohort achieved promising local control rates of 78% and overall survival of 85%; interestingly, all local failures occurred “within-field”. PRT was administered with minimal acute side effects (12). Additional comparative analysis of PRT and photon therapy for low-grade gliomas was assessed by Fuss et al. In their series, a dosimetric comparison was completed for seven pediatric patients with radiographic and/or clinically progressive optic nerve gliomas utilizing PRT planning, a lateral beam or wedge paired technique and 3D planned multi-field conformal treatment with a dose prescription of 54 Gy (13). PRT consistently reduced the volume of normal brain tissue encompassed by specified isodose lines. In comparison to lateral beam approach, PRT and, to a lesser extent, 3D photon planning demonstrated reduced radiation dose to D50% and D10% volumes of various organs at risk (e.g., contralateral optic nerve, optic chiasm, and pituitary) that lay in close proximity to the GTV. This approach has the strong potential to reduce long-term sequelae of irradiating normal tissues, an aspect that continues to be studied in ongoing trials of proton radiation therapy techniques. As further proton data matures, we will have a better understanding of the true potential to reduce late toxicity.
Medulloblastoma
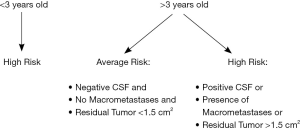
Medulloblastomas represent the most common non-glial malignant pediatric brain tumor. By definition, these tumors originate from the primitive neuroectoderm (PNET) arising from the cerebellum. PNET tumors represent a malignant group composed histologically of small round blue cells that are further subdivided into central PNET, of which medulloblastoma is an entity, neuroblastoma and peripheral PNET. Management of medulloblastoma depends on a patient’s risk classification as determined by various patient and tumor-related risk factors present at diagnosis; these factors account for risk of tumor recurrence. Average risk patients are those older than the age of 3 yrs without evidence of metastatic disease as evaluated by MRI brain and spine as well as lumbar cerebrospinal fluid (CSF) analysis that undergo gross or near total resection with <1.5 cm2 of residual tumor on a post-operative MRI scan. On the contrary, high-risk patients are those that do not fit these criteria noted for average risk classification (Figure 1).
Based on the results of the pilot study (CCG 9892) and subsequent phase III analysis (CCG/POG A9961) by Packer et al., current standard of care for average risk patients is craniospinal irradiation (CSI) to 23.4 Gy followed by posterior fossa boost to a dose of 54–55.8 Gy with concurrent weekly vincristine, and adjuvant chemotherapy to follow the completion of radiation therapy (14,15). Five yrs event-free survival rates of approximately 80% are achieved with this regimen, which are comparable to prior historical controls that utilized higher doses of CSI. Current medulloblastoma protocols are actively studying the ability to reduce CSI dose to 18 Gy, as well as compare the outcomes of posterior fossa boost vs. involved field boost in standard risk patients <8 yrs old (ACNS 0331).
As for patients with high-risk disease classification, current treatment approach recommendations include treatment with CSI to a dose of 36 Gy followed subsequently by posterior fossa (54–55.8 Gy) and metastatic site (39.6–54 Gy) boosts delivered concurrently with weekly vincristine. As with standard risk medulloblastoma patients, radiation therapy is followed by adjuvant chemotherapy with current protocols comparing the optimal concurrent and adjuvant chemotherapeutic regimens in this high-risk cohort (ACNS 0332).
For infants younger than 3 yrs old, who can comprise approximately 25–35% of all medulloblastoma cases, the intent of therapy is to delay delivery of radiation therapy, especially CSI, by using upfront chemotherapy to allow further neurocognitive development prior to utilization of radiation therapy; it is believed that most neural maturation occurs by age three. While in infants who have achieved a GTR without evidence of macroscopic metastatic disease (R0M0/M1) upfront chemotherapy can successfully delay utilization of radiation therapy as highlighted by Rutkowski et al. In those patients with residual disease and/or macroscopic metastatic disease (M2/M3), risk of progression is much higher and tends to occur early (16). As such, for this latter subset of infants, consideration of earlier radiation therapy vs. delaying radiation therapy with upfront chemotherapy use requires an individualized multidisciplinary approach that accounts for parents’ wishes as well as potential for increased neurocognitive side effect profile with delivery of both chemotherapy and radiation therapy.
With the advent of improving molecular technologies, the distinct genetic profiles of medulloblastomas are becoming more apparent with a resultant shift of incorporating this information into the risk stratification of ongoing clinical trials. Medulloblastomas can be categorized into four distinct molecular subgroups based on the presence or lack of various pathway alterations: wingless (WNT), sonic hedgehog (SHH), Myc-amplified group 3 and group 4 (9). WNT type medulloblastoma tend to have improved survival rates with standard of care. As a result, current protocols are assessing de-escalated radiation and chemotherapy for this subgroup of tumors. On the contrary, the SHH variant, which represents 25–30% of medulloblastoma tumors, can have variable prognosis. Various subtypes of SHH-MB exist that are differentiated by the underlying genetic mutation that results in over activation of the pathway, leading some subtypes to be sensitive to certain inhibitory therapies (i.e., SMO inhibitors, GLI inhibitors). These inhibitors (i.e., vismodegib) are being incorporated into ongoing protocols as adjuvant therapies to standard of care (17). Similarly, for group 3 medulloblastoma, previous studies have reported the potential efficacy of pemetrexed and gemcitabine in this subtype (18). As such, this had led to incorporation of these chemotherapeutics into adjuvant therapy for non-WNT, non-SHH medulloblastoma on current studies. With the increasing knowledge of the diverse genetic profile of medulloblastoma, the hope is to better stratify patients and therefore more accurately de-escalate or escalate tailored therapies in lieu of delivering the same approach of treating standard and high risk patients with the current treatment paradigm of surgical resection, radiation therapy and adjuvant chemotherapy.
Given the integral nature of CSI therapy to the treatment of medulloblastoma, and the potential long-term toxicities of this therapy, PRT has been considered for CSI delivery. CSI delivery with photon therapy can raise the potential risk of various long term sequelae in children (who as noted above can have long term survival outcomes); these potential toxicities include growth retardation secondary to vertebral body irradiation, cardiac dysfunction, primary hypothyroidism, or increased risk of second malignancies due to the exit beam of photon therapy that results in low dose being delivered to non-target sites. While alternative means have been proposed in an effort to reduce the risk of long term sequelae of CSI such as hyperfractionated dose regimens or electron therapy, inconsistent results and uncertainty of electron dose distribution have limited their adoption into current standard of care (19). PRT therefore has emerged as an enticing alternative therapeutic modality to reduce dose to normal tissues.
On the basis of prior data suggesting a correlation between dose delivery to the pancreas in children receiving irradiation and their eventual risk of developing diabetes mellitus, Brower et al. completed a dosimetric analysis of CSI approaches using 3D-CRT, IMRT using helical tomotherapy (IMRT-HT) and proton beam therapy (PBT) for five medulloblastoma patients to assess the ability of achieving pancreatic sparing (20). Both PRT and IMRT-HT were able to reduce not only mean dose to the whole pancreas and pancreatic tail, but also V5, V10 and V20 dose as compared to 3D-CRT. Additionally, PRT was further able to reduce mean dose to pancreas and pancreatic tail dose as well as V10 dose in comparison to IMRT-HT. By emphasizing the potential to reduce pancreatic dose with PRT, the authors highlight an important organ at risk to consider sparing in an effort to reduce the future risk of diabetes development in patients receiving current photon techniques.
Comparison of outcomes in a propensity matched analysis of standard risk medulloblastoma patients receiving CSI with posterior fossa/tumor bed boost using either proton or photon therapy was completed by Eaton et al. with the intention of closely monitoring long term incidence of endocrine abnormalities (21). The group of researchers closely followed a well-matched cohort of patients for incidence of hypothyroidism, growth hormone deficiency, adrenal insufficiency, sex hormone deficiency, precocious puberty, need for endocrine replacement therapy and height and body mass index standard deviation score (SDS). They observed that PBT remained a statically significant predictor of reduced risk of hypothyroidism, sex hormone deficiency, and need for endocrine therapy. Additionally, it was associated with greater height SDS in comparison to photon therapy in both multivariate and propensity matched analysis.
Similarly, models that predict for the risk of developing potential late effects have been developed that incorporate dose delivered to normal organs at risk with photon and PRT in an effort to more appropriately compare these treatment modalities. Brodin et al. studied ten pediatric patients with medulloblastoma evaluating treatment plans that incorporated CSI doses of 36 and 23.4 Gy followed by a posterior fossa boost to 54 Gy that were delivered with 3D-CRT, Rapid Arc IMRT, or IMPT; mean target doses were normalized to have the same value as the 3D-CRT plan to allow appropriate comparison of these techniques. The authors modeled the risk of late adverse events, as a function of age at radiation exposure and attained age. The estimate of solid second cancer risk was significantly lower with IMPT than for both photon techniques for 23.4 and 36 Gy prescribed CSI doses. The risk of developing late normal tissue complications, such as long-term pneumonitis, heart failure, xerostomia, blindness and ototoxicity risks were lower with PRT than photons (22). Zhang et al. similarly modeled the risk of secondary malignancy, incorporating age at irradiation, attained age, gender and dose delivered to radiosensitive organs to predict the risk between a photon and proton CSI plan prescribed to 23.4 Gy for a 4-yr-old medulloblastoma patient. Proton CSI predicted for a lower risk of secondary malignancy for all organs considered at all timepoints with a predicted lifetime risk of 11.6% with PRT and 138% (>100% due to ability to incur multiple malignancies) with photon CSI (23).
While PRT CSI has the potential for reduced late toxicity, it too has the ability to reduce acute toxicities. Brown et al. retrospectively analyzed adult medulloblastoma patients treated with either proton or photon CSI therapy. In a well-matched cohort, they found that proton CSI patients lost less weight than photon patients (median percent weight change of –1.2% and –5.8%, respectively, P=0.004). Fewer patients receiving PRT suffered weight loss of >5% from baseline than patients receiving photon therapy (P=0.004). Additionally, photon CSI had statistically significant higher rates of grade 2 nausea/vomiting, esophagitis management and bone marrow suppression (24).
In addition to the dosimetric benefit of protons for CSI delivery, there is a further benefit of reduced normal tissue irradiation with PRT when delivering the posterior fossa boost portion of medulloblastoma treatment; this point will be highlighted below in our discussion of PRT for infratentorial ependymomas.
Assessment of relapse patterns given the steep dose gradient achieved with PRT were analyzed by Sethi et al. for medulloblastoma patients, who were predominantly >3 yrs old and of standard risk classification (68%). With a median follow up of approximately 39 months, their analysis observed relapses in 16 patients of the cohort of 109 patients, a percentage not drastically different from the aforementioned event free survival rates with photon delivery (25). Ongoing trials of medulloblastoma treatments such as NCT01063114, which is evaluating use of proton CSI, will shed further light on the clinical outcomes and toxicity profile associated with this novel treatment technique.
Ependymoma
Ependymomas also represent a common entity that arises in the pediatric population, with a significant proportion of cases occurring in children younger than the age of five. While they have the ability to arise at any site in the ventricular system or spinal canal, they have a propensity to arise from the ependymal cells lining the fourth ventricle. While most tumors tend to extend locally, approximately 5–10% of tumors can present with diffuse leptomeningeal space involvement. Ependymomas are further subclassified as per the WHO grading system into myxopapillary ependymoma (WHO grade I), subependymoma (WHO grade I), classical ependymoma (WHO grade II), and anaplastic ependymoma (WHO grade III). Initial treatment modality of choice for intracranial ependymoma is maximal safe resection, which is challenging due to the proximity and the adherence of the tumor to cranial nerves and the brain stem. Postoperative RT is recommended in all cases of posterior fossa ependymomas even after a GTR. Adjuvant radiation therapy is warranted with statistically improved local control in this subset of children with a trend towards improved survival (26). For children with supratentorial non-anaplastic ependymomas after GTR, small series report favorable outcomes with deferment of upfront RT, though this paradigm certainly requires further support (27). The role of RT volume has also developed over time, with the current recommendation of involved field radiation therapy to the GTV +1 cm margin respecting surrounding normal tissue boundaries shown to achieve acceptable local control rates (3 yrs local failure rate: 15%) in comparison to historical series (28). While the series revealed a higher than expected rate of distant relapse, this was felt to be in part due to potential anaplastic histology as well as significantly higher rates of GTR which may have changed recurrence patterns. Additionally, Merchant et al. highlights the importance of upfront RT even in the youngest cohort; patients who received adjuvant chemotherapy in an effort to delay radiation therapy had a worse progression free survival rate than those patients receiving radiation immediately following resection (3 yrs PFS 60% vs. 78% respectively, P=0.0446). Similarly, Grundy et al. used chemotherapeutic protocols after surgical resection in children <3 yrs old with RT only for salvage therapy. In their series, while 5 yr OS was 64% in patients presenting with non-metastatic intracranial ependymoma, only 42% of patients at 5 yrs were able to truly avoid radiation therapy, thereby resulting in administration of both chemotherapy and radiation therapy in a large proportion of patients which could potentiate the risk for long term neurotoxicity (29). A short course of chemotherapy is currently recommended after STR in an attempt to achieve GTR with a second-look surgery followed by RT.
Despite the trend for increasing conformality of radiation delivery, involved field radiation still results in dose spill over to normal organs at risk, such as the brainstem, hypothalamus, pituitary, cochlea, hippocampi and normal brain parenchyma which raises the potential for long term cognitive, neurological and endocrine dysfunction as result of this increased dose bath. As such, multiple single institution series have attempted to show the potential benefit of using PBT in reducing dose to these structures. Mizumoto et al. analyzed the potential reduction of normal brain parenchyma irradiation in six patients with intracranial ependymoma who were treated with PBT by completing a dosimetric comparison to photon conformal RT for these patients’ plans (30). Their study revealed that utilization of PRT resulted in a median decrease of mean normal brain dose by 47%. By utilizing a theoretical model created by Merchant et al. that predicts for IQ deterioration after RT delivery and incorporates mean normal brain dose as a parameter in the model, Mizumoto et al. predicted that using proton beam could reduce the IQ decrease by approximately half of what is predicted for photon therapy.
MacDonald et al. have also reported one of the largest series of intracranial ependymoma patients treated with PRT (31). In their series, 70 patients underwent involved field PRT with most patients receiving 54 GyE (RBE) delivered in 1.8 GyE (RBE). With a median follow up of approximately 46 months, they reported a 3 yrs PFS rate of 76% and 3 yrs OS rate of 95%, comparable to photon based series. They noted 5 yrs local and distant control rates of 77% and 83%, respectively. Additionally, they evaluated subsets of their patients for assessment of long-term endocrinopathies, auditory and neurocognitive changes. They found few reports of growth hormone deficiency or hypothyroidism. Similarly, in the 27 patients who had long term audiology evaluation, only two patients suffered hearing deficits with both patients having tumors that extended to the foramen of Luschka therefore resulting in high cochlear doses than that received by the remainder of the cohort; average median cochlear doses among 68 patients was 7.1 and 6.95 GyE (RBE) for right and left cochlea, respectively. Lastly, their analysis of neurocognitive testing revealed that with a follow up time of approximately 2 yrs, in the subset of patients who completed testing there was no deterioration of IQ or adaptive skills/functional independence after RT in comparison to pre-RT scores.
As the utilization of PBT increases in the treatment of intracranial ependymomas, Gunther et al. raise an important consideration regarding the need for close follow up in these patients due to the potential for MRI imaging changes that can occur. In their retrospective analysis of 72 patients (35 IMRT, 37 PRT) they followed treated patients (median dose: 59.4 Gy PRT, 54 Gy IMRT) with serial MRI and graded imaging changes based on a pre-established scale. The twenty-two patients (6 IMRT, 16 PRT) who exhibited MRI changes, mostly T2 hyperintensity and T1 enhancement, were younger at diagnosis and at time of radiation therapy, began radiation earlier after surgery and also received a higher median dose. For those patients with infratentorial ependymomas treated with PRT, presence of imaging changes correlated with higher median D50% to brainstem (median D50%: 56 Gy for patients with changes vs. 42.8 Gy for no changes, P=0.02). Overall, multivariate factors that significantly correlated with higher rate of imaging changes included use of PRT (OR: 3.89, P=0.024), shorter interval between surgery and radiation, and age <3 yrs. Clinical outcomes data revealed no difference in survival between patients receiving PRT or IMRT (4 yrs OS: 87.5% vs. 78.8%, respectively, P=0.21) or in outcomes amongst patients with changes or no changes (4 yrs OS: 90.4% vs. 82%, respectively, P=0.56) (32). Seven patients (3 IMRT, 4 PRT) had symptomatic MRI changes, which were predominantly treated with steroids, bevacizumab and/or hyperbaric oxygen. While the group was unable to identify factors that predicted for symptomatic MRI changes, they did note that ongoing analyses will further study this topic. As Gunther et al. emphasize, it is important with the use of PRT to be cognizant of the RBE uncertainty, especially in the region of the spread-out Bragg peak (SOBP), as it may lead to increasing imaging changes that future use of PRT will reveal. The true clinical significance of these imaging finding remains to be elucidated.
Conclusions
As exemplified by the EUROCARE cancer registry project which studies trends over time of childhood cancer survival rates, risk of dying from pediatric CNS tumors, PNET/medulloblastomas and astrocytomas decreased by 3% per yr from 1983–1994; such as trend is present not only in Europe but also in the USA (33). With the advent of improved treatment modalities, our goal for treating pediatric malignancies should be not only to increase survival rates, but also quality of life for these survivors (34). PRT is an important modality that can help achieve such a profound goal. As highlighted in this review, utilization of PRT has been considered since as early as the 2000’s for the most common childhood CNS malignancies. It has maintained continued interest because it not only shows comparable clinical outcomes to photon therapy but also the dosimetric advantage of reducing dose to unintentionally irradiated tissues. As increasing number of proton centers begin to open, and a greater number of children are accrued to receiving PRT, the ultimate hope is to complete a well-controlled prospective trial comparing proton and photon radiation therapy. In the interim, PRT should be strongly considered when treating pediatric CNS tumors in an effort to allow children to live and mature with minimal treatment sequelae.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol 2014;16 Suppl 4:iv1-63. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Schwartz CL. Long-term survivors of childhood cancer: the late effects of therapy. Oncologist 1999;4:45-54. [PubMed]
- Chalil A, Ramaswamy V. Low Grade Gliomas in Children. J Child Neurol 2016;31:517-22. [Crossref] [PubMed]
- Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol 2009;24:1397-408. [Crossref] [PubMed]
- Fisher PG, Tihan T, Goldthwaite PT, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer 2008;51:245-50. [Crossref] [PubMed]
- Fisher BJ, Leighton CC, Vujovic O, et al. Results of a policy of surveillance alone after surgical management of pediatric low grade gliomas. Int J Radiat Oncol Biol Phys 2001;51:704-10. [Crossref] [PubMed]
- Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg 1997;86:747-54. [Crossref] [PubMed]
- Gajjar A, Bowers DC, Karajannis MA, et al. Pediatric Brain Tumors: Innovative Genomic Information Is Transforming the Diagnostic and Clinical Landscape. J Clin Oncol 2015;33:2986-98. [Crossref] [PubMed]
- Yalon M, Rood B, MacDonald TJ, et al. A feasibility and efficacy study of rapamycin and erlotinib for recurrent pediatric low-grade glioma (LGG). Pediatr Blood Cancer 2013;60:71-6. [Crossref] [PubMed]
- Merchant TE, Kun LE, Wu S, et al. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol 2009;27:3598-604. [Crossref] [PubMed]
- Hug EB, Muenter MW, Archambeau JO, et al. Conformal proton radiation therapy for pediatric low-grade astrocytomas. Strahlenther Onkol 2002;178:10-7. [Crossref] [PubMed]
- Fuss M, Hug EB, Schaefer RA, et al. Proton radiation therapy (PRT) for pediatric optic pathway gliomas: comparison with 3D planned conventional photons and a standard photon technique. Int J Radiat Oncol Biol Phys 1999;45:1117-26. [Crossref] [PubMed]
- Packer RJ, Goldwein J, Nicholson HS, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children's Cancer Group Study. J Clin Oncol 1999;17:2127-36. [PubMed]
- Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 2006;24:4202-8. [Crossref] [PubMed]
- Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med 2005;352:978-86. [Crossref] [PubMed]
- Ransohoff KJ, Sarin KY, Tang JY. Smoothened Inhibitors in Sonic Hedgehog Subgroup Medulloblastoma. J Clin Oncol 2015;33:2692-4. [Crossref] [PubMed]
- Morfouace M, Shelat A, Jacus M, et al. Pemetrexed and gemcitabine as combination therapy for the treatment of Group3 medulloblastoma. Cancer Cell 2014;25:516-29. [Crossref] [PubMed]
- Miralbell R, Lomax A, Russo M. Potential role of proton therapy in the treatment of pediatric medulloblastoma/primitive neuro-ectodermal tumors: spinal theca irradiation. Int J Radiat Oncol Biol Phys 1997;38:805-11. [Crossref] [PubMed]
- Brower JV, Gans S, Hartsell WF, et al. Proton therapy and helical tomotherapy result in reduced dose deposition to the pancreas in the setting of cranio-spinal irradiation for medulloblastoma: implications for reduced risk of diabetes mellitus in long-term survivors. Acta Oncol 2015;54:563-6. [Crossref] [PubMed]
- Eaton BR, Esiashvili N, Kim S, et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol 2016;18:881-7. [Crossref] [PubMed]
- Brodin NP, Munck Af Rosenschöld P, Aznar MC, et al. Radiobiological risk estimates of adverse events and secondary cancer for proton and photon radiation therapy of pediatric medulloblastoma. Acta Oncol 2011;50:806-16. [Crossref] [PubMed]
- Zhang R, Howell RM, Giebeler A, et al. Comparison of risk of radiogenic second cancer following photon and proton craniospinal irradiation for a pediatric medulloblastoma patient. Phys Med Biol 2013;58:807-23. [Crossref] [PubMed]
- Brown AP, Barney CL, Grosshans DR, et al. Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys 2013;86:277-84. [Crossref] [PubMed]
- Sethi RV, Giantsoudi D, Raiford M, et al. Patterns of failure after proton therapy in medulloblastoma; linear energy transfer distributions and relative biological effectiveness associations for relapses. Int J Radiat Oncol Biol Phys 2014;88:655-63. [Crossref] [PubMed]
- Rogers L, Pueschel J, Spetzler R, et al. Is gross-total resection sufficient treatment for posterior fossa ependymomas? J Neurosurg 2005;102:629-36. [Crossref] [PubMed]
- Hukin J, Epstein F, Lefton D, et al. Treatment of intracranial ependymoma by surgery alone. Pediatr Neurosurg 1998;29:40-5. [Crossref] [PubMed]
- Merchant TE, Mulhern RK, Krasin MJ, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol 2004;22:3156-62. [Crossref] [PubMed]
- Grundy RG, Wilne SA, Weston CL, et al. Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol 2007;8:696-705. [Crossref] [PubMed]
- Mizumoto M, Oshiro Y, Takizawa D, et al. Proton beam therapy for pediatric ependymoma. Pediatr Int 2015;57:567-71. [Crossref] [PubMed]
- Macdonald SM, Sethi R, Lavally B, et al. Proton radiotherapy for pediatric central nervous system ependymoma: clinical outcomes for 70 patients. Neuro Oncol 2013;15:1552-9. [Crossref] [PubMed]
- Gunther JR, Sato M, Chintagumpala M, et al. Imaging Changes in Pediatric Intracranial Ependymoma Patients Treated With Proton Beam Radiation Therapy Compared to Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys 2015;93:54-63. [Crossref] [PubMed]
- Gatta G, Capocaccia R, Stiller C, et al. Childhood cancer survival trends in Europe: a EUROCARE Working Group study. J Clin Oncol 2005;23:3742-51. [Crossref] [PubMed]
- Armstrong FD. Proton-beam radiation therapy and health-related quality of life in children with CNS tumors. J Clin Oncol 2012;30:2028-9. [Crossref] [PubMed]