A review of hepatocellular carcinoma (HCC) staging systems
Introduction
Hepatocellular carcinoma (HCC) is the third-leading cause of cancer death worldwide. Despite its enormous global impact, there is much disagreement about how best to stage and characterize this cancer. The differences in approach to HCC are due in part to its inherent clinical and biologic heterogeneity, but are also a function of the prism through which clinicians and clinical researchers observe the cancer. Despite numerous validation and comparative studies, and “consensus” panel recommendations generated by hepatologists, oncologists, surgeons and radiologists, with varying degrees of multidisciplinary collaboration, there is still no single system that could be called the “standard” for classifying HCC.
Like with any cancer, the goals of a tumor staging system in HCC are to estimate a patient’s prognosis, which allows for appropriate therapy to be selected. The identification of that appropriate therapy, in turn, requires a staging paradigm that standardizes the platform for researchers to exchange data regarding treatments and outcomes (1). Ideally, and most challenging with HCC, staging systems should assure balance of important prognostic factors across treatment arms within a clinical trial population to avoid confounding of outcomes by baseline differences.
The task of accounting for the heterogeneity of HCC is not only a reflection of the different viral or metabolic conditions at the root of the cancer, but also of the extent of impaired liver function. The challenge of measuring the contributions of the cancer and hepatic dysfunction to the overall prognosis was recognized with the first modern-era liver cancer staging system, which was proposed at the Hepatocellular Carcinoma International Symposium in Kampala, Uganda in 1971 (2). Subsequent attempts at HCC staging have continued to employ both tumor and liver-specific variables in the setting where there is often very limited diagnostic tissue, which means that there may be no information from a pathological examination. This reflects the fact that biopsy may not be a pre-requisite to diagnosis of HCC (3). Serum alpha-fetoprotein (AFP) is a commonly-used screening biomarker in patients at risk for HCC but is not sufficient for surveillance or diagnosis due to lack of sensitivity and specificity (4). Although retrospective data have established high AFP at presentation as a negative prognostic factor, serum AFP level is included in only a subset of HCC staging systems (Table 1).
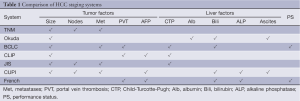
Full table
For a staging system to be effective and widely used, it has to be reliable, reproducible and simple, using data elements that can be obtained as part of standard clinical practice across a wide range of treatment sites. Most HCC staging systems have identified prognostic factors through multivariate analyses of large cohorts of patients to weight the different variables according to prognostic impact. Once proposed, a classification system must be validated across the spectrum of HCC cohorts.
We will first review the principal system used to score underlying liver function in cirrhotic patients, the Childs-Turcotte-Pugh score (CTP). Next we consider the Model for End-Stage Liver Disease (MELD), which predicts short-term prognosis and is extensively used in liver transplant evaluation. We then examine seven commonly-utilized HCC staging systems with respect to their development and limitations. Finally, we will look ahead to novel molecular and biomarker-based staging systems which we hope will enable us to refine our understanding and classification of this complex and heterogeneous cancer.
Child-Turcotte-Pugh (CTP)
The prognostic importance of liver function was first codified in the Child-Turcotte publication in 1964 (5), where patients being considered for surgery for portal venous shunting were risk-stratified into three categories. The initial Child-Turcotte staging included clinical assessments of encephalopathy, ascites, nutritional status and laboratory measurements of serum bilirubin and albumin and then was modified by Pugh in 1973 (6), with the replacement of nutritional status by prothrombin time (Table 2).

Full table
The CTP score is the simplest and most widely used grading system for liver function. Given that most HCCs arise in the milieu of cirrhosis, and surgical interventions have the highest potential of cure, CTP is ubiquitous in the evaluation of HCC. In addition to routine clinical and research use, the CTP score is referenced routinely by regulatory agencies reviewing new drug applications. However, the drawbacks are many, including inter-laboratory variations, day-to-day fluctuations in the key parameters and the subjective nature of the clinical grading of encephalopathy and ascites (7). Though the CTP score by itself does not include any HCC-specific parameters, it has been incorporated into multiple contemporary scoring systems including Cancer of the Liver Italian Program (CLIP) and Barcelona Clinic Liver Cancer (BCLC).
Model for end stage liver disease (MELD)
The MELD score, initially developed to determine prognosis following a transjugular intra-hepatic shunt (TIPS) procedure for liver failure (8), is now widely used in the liver transplant arena to prioritize donor liver allocation. It is a logarithmic score that is comprised of International Normalized Ratio (INR), serum creatinine, total serum bilirubin and the etiology of cirrhosis. After minor modifications, the resulting MELD model, which has been validated in 4 independent populations (9), can be generalized to all patients with end-stage liver disease.
A modification of the MELD score formula (Figure 1), with the variable for etiology of cirrhosis excluded, was adopted by the United Network of Organ Sharing (UNOS) in February 2002 as the standard by which transplant recipients are prioritized. Given that a higher score is associated with shorter survival, priority for receipt of a transplant is logical. The implementation of MELD led to reduction in registration for the waiting list and mortality while on the list (10), as well as reduced median waiting time to liver transplantation (11).
The strength of the MELD score is its prediction of short-term mortality, and is therefore able to identify the “sickest” patients for graft allocation. However, it fails to correctly classify a portion of patients with advanced cirrhosis (12), and several groups have offered refinements to the score (13-15).
Selected patients with HCC may be appropriate candidates for a curative orthotopic liver transplant (16,17). However, patients with early stage HCC but compensated liver disease may suffer cancer progression while waiting for their MELD score to move them up on the graft allocation priority list. This has been “remedied” by awarding extra points to the MELD score for a diagnosis of HCC; while this has been shown to improve the likelihood of timely transplant in these patients (18), the tilt towards allocating livers to patients who could succumb to the malignancy has been debated (19).
Overview of current staging systems
TNM
No cancer would be complete without a TNM staging algorithm. The criteria are developed jointly by the American Joint Committee on Cancer (AJCC) and the International Union for Cancer Control (UICC) and have been updated regularly since the first edition in 1977; the Seventh Edition took effect in 2010 (1).
The TNM system assesses primary tumor features (T), the, presence or absence of nodal involvement (N) and distant metastasis (M). Additional information which may be included are the histologic grade (G) and fibrosis score (F) based on the Ishak classification (20), but these factors do not affect staging (Table 3).
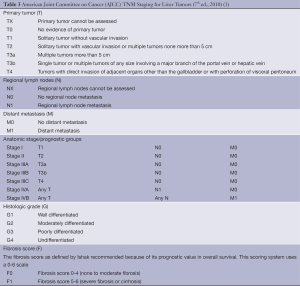
Full table
Recent versions of the TNM staging have been influenced largely by data from patients who underwent curative resections. In 2002, Vauthey et al. proposed a simplification of the TNM, after stratifying the survival of 557 patients who underwent resections. They recommended that the T-component focus on vascular invasion, tumor number and tumor size (21). In a similar analysis of surgical patients in Hong Kong, with a predominance of hepatitis B, Poon and Fan found the key prognostic factors for 5-year survival are major vascular invasion, microvascular invasion and involvement of surrounding tissues (22).
In essence, the TNM system is based on histopathology and is applicable in prognosticating survival for the distinct minority of patients who have undergone curative surgery. By itself, the TNM T-stage does not offer guidance on resectability and therefore adds very little discriminatory value to patient assessment. It has little relevance to patients presenting with advanced disease because of the model’s inability to reflect the prognosis of underlying liver disease.
Okuda score
The Okuda system is a prognostic score introduced in 1985 (23) and incorporates both tumor features as well as the degree of underlying cirrhosis. Using a cohort of 850 patients with an unequivocal diagnosis of HCC between 1975-1983, Okuda and colleagues devised a staging system based on four factors representing advanced disease. This includes tumor occupying greater or less than 50% of the liver, the presence or absence of ascites, and serum albumin and bilirubin levels (Table 4). In the original cohort, median survival was 11.5 months for Stage I, 3.0 months for Stage II and 0.9 months for Stage III.
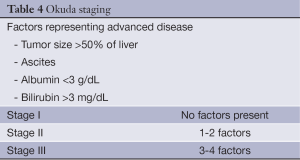
Full table
Because many in the index population (38.5-45%) died of liver failure, the system emphasizes underlying liver dysfunction.
Despite not having been prospectively evaluated, the Okuda system is still in use, but with the evolution of imaging and surveillance, it is the extraordinary patient whose tumor is not discovered well before it occupies more than half the liver. The system’s biggest shortcoming is its relatively crude classification of early stage patients and subsequent staging systems have tried to better characterize Okuda Stage I patients. Contemporary models have all adopted the practice of including liver-specific variables and some have even incorporated the Okuda score into newer formulae. Indeed, the Okuda system remains the standard against which newer scoring systems are compared.
BCLC staging classification
The BCLC classification was first published in 1999 (24) and is considered the standard HCC system by the American Association of for the Study of Liver Disease (AASLD) (4) and European Association for the Study of the Liver (25). These endorsements and the substantial contributions to HCC research by the hepatologists who described BCLC sometimes disguise the reality that not every clinician and researcher in the field agrees with the stance of the distinguished liver societies.
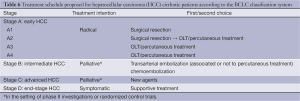
Derived from a single institution experience, BCLC takes into account size and extent of the primary tumor, liver function and physiological factors and incorporates the Okuda stage and Child-Pugh score (Table 5). There is a corresponding treatment schedule for each stage (Table 6), ranging from curative therapies such as resection or transplant for early stage patients to best supportive care for end-stage patients. Prospective and retrospective studies on Italian cohorts (26-28), in which the majority of patients underwent radical therapies, found BCLC to be a better prognostication system compared to the other commonly used systems. Marrero et al. reported in 2005 that, in a cohort of 239 consecutive American patients seen at the University of Michigan Medical Center’s Liver Clinics, BCLC had the best prognostic stratification when compared to 6 other commonly used staging systems (29). While other investigators have failed to come to the same conclusion (30-33), BCLC has gained widespread popularity since its introduction.
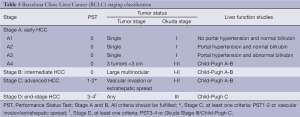
Full table
Full table
More controversial than the prognostic scoring system is the treatment algorithm that is a part of the BCLC. It lacks discrimination within the intermediate stage (BCLC-B) patients, a large proportion of the HCC population. The burden of liver disease which falls under BCLC stage B can vary greatly, from four small tumors to near complete replacement of the liver by tumor, provided liver function is preserved and there is no vascular invasion, extrahepatic spread, or compromised performance status, which would upstage to BCLC stage C or D. Consequently, in practice, some BCLB-B patients may no longer be eligible for liver-directed therapies, and are generally treated following BCLC-C algorithms. The heterogeneity within the BCLC-B classification also introduces the potential for prognostic heterogeneity within clinical research protocols employing BCLC stage for eligibility or stratification.
CLIP score
The CLIP score was proposed in 1998 and by incorporating Child-Pugh stage, tumor morphology, AFP level and the presence or absence of portal vein thrombosis, takes into account both liver function and tumor characteristics (34) (Table 7). However, what constitutes “massive” is subjective, without specific size criteria.

Full table
To derive the score, a retrospective analysis was performed between 1990-1992 of 435 HCC consecutive patients, almost all with cirrhosis, presenting to the 16 CLIP institutions. Univariate analysis identified significant predictors of overall survival, and these were included into a stratified Cox proportional hazards regression model, with loco-regional therapy as the stratification factor. The majority of patients (56.8%) received some form of loco-regional treatment and only a few (2.7%) underwent surgery.
The CLIP score (range from 0-5) was first validated by the original investigators on a prospective cohort of 196 HCC patients with cirrhosis being enrolled in a clinical trial (35) and has subsequently been validated on Japanese, Canadian and German cohorts of patients (36-38). CLIP was found to be a good predictor of recurrence in a retrospective analysis of a Chinese cohort of 174 predominantly Hepatitis B positive patients with HCC who underwent curative resection (39). The CLIP score also performed better than other prognostication systems when used to retrospectively analyze 131 Korean patients, with unresectable HCC, who were undergoing transarterial chemoembolization (TACE) (40).
The CLIP score is not flawless. The paucity of patients undergoing curative surgery in the original cohort may limit its ability to prognosticate early stage patients. Although a retrospective analysis of patients in Canada (37), 28% of whom underwent surgery, CLIP was found to be superior to Okuda in identifying early stage patients with a good prognosis, it is not as accurate at the JIS (see below). However, other investigators have suggested the CLIP is comparatively superior to contemporary systems (41,42) and may be further improved by the inclusion of performance status (42).
Japan integrated staging (JIS)
In 2003, the The Liver Cancer Study Group of Japan (LCSGJ) proposed the JIS score (43). Arguing that the CLIP score, previously validated in a Japanese population (36), did not provide sufficiently accurate prognostication for the early stage patients commonly diagnosed in Japanese centers due to screening programs and increased awareness of HCC, these investigators directed their efforts towards emphasizing the very favorable group from other early-stage patients.
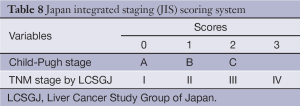
The JIS score was developed from a cohort 722 consecutive Japanese patients and appears superior at prognosticating survival compared to CLIP, particularly in patients with early stage disease. The JIS system incorporates the LCSGJ’s modification of the TNM system and the Child-Pugh score (Table 8). Patients with a JIS score of 0 had a 10-year survival rate of 65% while patients with a CLIP score of 0 had 10-year survival rates of only 23%.
Full table
While it has been validated in Japan (44,45) and in other Asian populations, the JIS has not been prospectively validated in a Western population. There have been attempts to modify the JIS (46), as well as to incorporate biomarkers like AFP into the system (47,48); these versions have also not been validated and have not gained traction outside of Japan.
Chinese University Prognostic Index (CUPI)
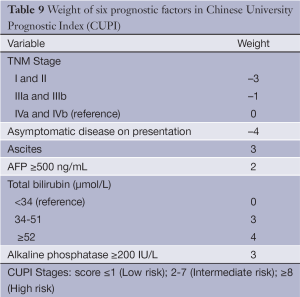
The CUPI was developed at a single center in Hong Kong based on a retrospective analysis of 926 ethnic Chinese patients (49). As expected, based on the population’s demographics, the cohort had a high proportion with hepatitis B (79%). The cohort was also predominantly male (83%) and the majority (58.4%) of patients were too advanced to receive any surgery or interventional therapy. A Cox regression model was constructed containing TNM staging followed by forward stepwise addition of 18 other relevant clinical variables. The outcome measurement was death within 3 months of diagnosis. In addition to confirming TNM staging as a highly significant predictor of 3-month survival, the model identified presentation with asymptomatic disease, AFP level, total bilirubin, serum alkaline phosphatase and clinical detection of ascites as significant prognostic factors (Table 9).
Full table
The original investigators were able to prospectively validate CUPI in a group of 595 largely hepatitis-B positive Asians (50). The CUPI is well-designed and easy to use. The weighted scoring system in CUPI is more refined than the rather blunt assignment of points in CLIP and JIS. CUPI is derived from a cohort which is predominantly hepatitis B and performs well in similar Asian populations. Of note, 2 recent studies have found that CUPI, as well as the CLIP score, are the best models to predict survival in patients with advanced HCC enrolling in clinical trials for systemic therapy at Asian centers (33,51). However, it has not performed well in comparative studies in Western populations, which are characterized by a greater proportion of patients with hepatitis C.
Groupe d¡¯Etude et de Traitement du Carcinome H¨¦patocellulaire (GRETCH)
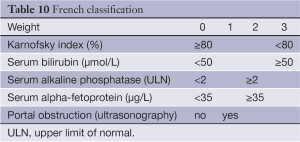
The French scoring system, proposed by GRETCH in 1999 (52), uses objective measures and an estimate of performance status to predict survival. A cohort of 761 consecutive patients across 24 institutions in Europe and Canada were randomly assigned to a training sample (506 patients) or a validation sample (255 patients.) Predictors of survival were identified using univariate analysis with Kaplan-Meier estimates and then included in a Cox proportional hazards model. Using a forward stepwise selection, five factors were found to affect 1-year survival from the time of diagnosis. These are performance status by Karnofsky score, serum bilirubin, serum alkaline phosphatase, AFP, and presence or absence of portal obstruction by ultrasonography (Table 10).
Full table
An advantage of the French classification is that its variables are generally available at the time of initial diagnosis and do not require invasive procedures or sophisticated imaging. The increasing use of cross-sectional imaging as a diagnostic modality could impact the prognostic value of this scoring system by altering the sensitivity for diagnosis of portal obstruction. To date, however, this classification system has not improved prognostic discrimination in comparison to other systems when tested on various cohorts (26,42,53).
Limitations of current staging systems
The heterogeneous nature of HCC has made it difficult to implement a universally accepted staging system. While the various systems emphasize to a different degree the importance of tumor characteristics and liver function (Table 1), none of the classification algorithms account for location of the tumor or its proximity to major vessels. In turn, the tempo of the deterioration of the underlying liver disease is also difficult to calculate, both because of the risk of worsening cirrhosis if it exists or the proclivity for central HCC tumors to invade the portal vein. Frequently, patients can be clinically stable for an extended period of time before experiencing decompensated liver failure. With serial liver function tests and imaging, clinicians hope to recognize impending signs of liver failure.
Finally, the underlying risk factors and the complex tumor biology of HCC are not accounted for by any of these systems. Many studies describe differences in cancer outcomes based on the etiology of cirrhosis. For example, hepatitis C patients and patients with alcoholic liver disease generally experience poorer outcomes than HBV-positive patients undergoing resection (54,55), which is generally attributed to the propensity of some HBV-associated HCCs to bypass the premalignant state of cirrhosis. Conversely, post-hoc subset analyses suggest that HCV and alcoholic liver disease HCC subgroups experience better outcomes with sorafenib therapy (56). An increasing number of patients now develop HCC secondary to underlying non-alcoholic fatty liver disease (NAFLD), which also may impact prognosis (57). These examples highlight the challenges of discriminating the prognostic impact of the extent and etiology of underlying liver disease from that of tumor factors such as stage and tumor biology.
Novel staging systems
With emerging understanding of HCC genomics, it is now apparent that common molecular subclasses exist which are associated with prognosis, may be enriched in certain subsets according to etiology of liver disease, and which could impact response to targeted therapies (58,59). In this clinically- and genomically-complex disease, it is likely that tumor biology will play an important role in future staging. Several recently proposed staging systems, which incorporate molecular biomarkers—of both tumor and cirrhosis—are discussed below.
Genomic signatures
Over the past decade, numerous molecular signatures have been proposed to predict recurrence and cancer outcomes in surgically resected HCC (58,60). In 2011, Villanueva et al. evaluated 22 different molecular signatures and identified 2—the G3 signature from tumor and the poor-survival signature from adjacent nontumoral cirrhotic tissue—which, together with clinico-pathological features, were associated with recurrence (61).
5-gene score
Recently, a gene expression score has been proposed to predict disease-specific survival and early tumor recurrence of resected HCC (62). 5 genes (TAF9, RAMP3, HN1, KRT19 and RAN) were selected for their prognostic value in a French cohort. Patients were stratified into good and poor risk groups and the authors applied the gene score to several independent cohorts.
IGF-modified CTP staging
Serum insulin-like growth factor-1 (IGF-1) has been proposed as a surrogate for hepatic function because its production is reduced in cirrhosis (63).
Conclusions
The perfect unifying HCC staging system does not exist, nor is one necessary. Striving to better characterize and classify this disease remains a worthy endeavor, particularly if we are able to identify subsets of patients who garner substantial benefit from interventions. Depending upon the direction in which the field moves, we may be discussing entirely different systems a few years from now.
Accurately staging a disease and stratifying patients in clinical trials is not the same as correctly managing it. Because of its widespread presence in contemporary HCC research, BCLC is used by many practitioners to guide clinical decision-making. While this is certainly reasonable, and lays the framework for investigators and treating physicians alike to make best use of current data in treating a difficult cancer, it should not be taken as evidence that BCLC is the most accurate or refined system.
On the horizon, our growing understanding of the complex tumor biology in HCC along with novel imaging techniques and advances in the management of viral hepatitis and cirrhosis herald a new era of staging and scoring systems. As a complement to clinical staging, it is certainly to be hoped that these emerging systems will allow us to improve our prognostic ability and deliver more effective care to patients with HCC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- AJCC Cancer Staging Handbook. 7 ed. Chicago, IL: American Joint Committee on Cancer, 2010.
- Vogel CL. International Symposium on Hepatocellular Carcinoma—Kampala, Uganda (July 1971). JNCI J Natl Cancer Inst 1972;48:567-71.
- Benson AB 3rd, Abrams TA, Ben-Josef E, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw 2009;7:350-91. [PubMed]
- Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [PubMed]
- Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg 1964;1:1-85. [PubMed]
- Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. [PubMed]
- Botta F, Giannini E, Romagnoli P, et al. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study. Gut 2003;52:134-9. [PubMed]
- Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000;31:864-71. [PubMed]
- Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464-70. [PubMed]
- Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003;124:91-6. [PubMed]
- Wiesner R, Lake JR, Freeman RB, et al. Model for end-stage liver disease (MELD) exception guidelines. Liver Transpl 2006;12:S85-7. [PubMed]
- Al Sibae MR, Cappell MS. Accuracy of MELD scores in predicting mortality in decompensated cirrhosis from variceal bleeding, hepatorenal syndrome, alcoholic hepatitis, or acute liver failure as well as mortality after non-transplant surgery or TIPS. Dig Dis Sci 2011;56:977-87. [PubMed]
- Biggins SW, Kim WR, Terrault NA, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 2006;130:1652-60. [PubMed]
- Huo TI, Lee SD, Lin HC. Selecting an optimal prognostic system for liver cirrhosis: the model for end-stage liver disease and beyond. Liver Int 2008;28:606-13. [PubMed]
- Somsouk M, Kornfield R, Vittinghoff E, et al. Moderate ascites identifies patients with low model for end-stage liver disease scores awaiting liver transplantation who have a high mortality risk. Liver Transpl 2011;17:129-36. [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [PubMed]
- Yao FY, Bass NM, Ascher NL, et al. Liver transplantation for hepatocellular carcinoma: lessons from the first year under the Model of End-Stage Liver Disease (MELD) organ allocation policy. Liver Transpl 2004;10:621-30. [PubMed]
- Roberts JP, Venook A, Kerlan R, et al. Hepatocellular carcinoma: Ablate and wait versus rapid transplantation. Liver Transpl 2010;16:925-9. [PubMed]
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696-9. [PubMed]
- Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol 2002;20:1527-36. [PubMed]
- Poon RT, Fan ST. Evaluation of the new AJCC/UICC staging system for hepatocellular carcinoma after hepatic resection in Chinese patients. Surg Oncol Clin N Am 2003;12:35-50. [PubMed]
- Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 1985;56:918-28. [PubMed]
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [PubMed]
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [PubMed]
- Cillo U, Bassanello M, Vitale A, et al. The critical issue of hepatocellular carcinoma prognostic classification: which is the best tool available? J Hepatol 2004;40:124-31. [PubMed]
- Cillo U, Vitale A, Grigoletto F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol 2006;44:723-31. [PubMed]
- Vitale A, Saracino E, Boccagni P, et al. Validation of the BCLC prognostic system in surgical hepatocellular cancer patients. Transplant Proc 2009;41:1260-3. [PubMed]
- Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology 2005;41:707-16. [PubMed]
- Georgiades CS, Liapi E, Frangakis C, et al. Prognostic accuracy of 12 liver staging systems in patients with unresectable hepatocellular carcinoma treated with transarterial chemoembolization. J Vasc Interv Radiol 2006;17:1619-24. [PubMed]
- Chung H, Kudo M, Takahashi S, et al. Comparison of three current staging systems for hepatocellular carcinoma: Japan integrated staging score, new Barcelona Clinic Liver Cancer staging classification, and Tokyo score. J Gastroenterol Hepatol 2008;23:445-52. [PubMed]
- Chen CH, Hu FC, Huang GT, et al. Applicability of staging systems for patients with hepatocellular carcinoma is dependent on treatment method--analysis of 2010 Taiwanese patients. Eur J Cancer 2009;45:1630-9. [PubMed]
- Li X, Dong M, Lin Q, et al. Comparison of current staging systems for advanced hepatocellular carcinoma not amendable to locoregional therapy as inclusion criteria for clinical trials. Asia Pac J Clin Oncol 2013;9:86-92. [PubMed]
- A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28:751-5. [PubMed]
- Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology 2000;31:840-5. [PubMed]
- Ueno S, Tanabe G, Sako K, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology 2001;34:529-34. [PubMed]
- Levy I, Sherman M, Liver Cancer Study Group of the University of Toronto. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging systems in a cohort of 257 patients in Toronto. Gut 2002;50:881-5. [PubMed]
- op den Winkel M, Nagel D, Sappl J, et al. Prognosis of patients with hepatocellular carcinoma. Validation and ranking of established staging-systems in a large western HCC-cohort. PLoS One 2012;7:e45066.
- Zhao WH, Ma ZM, Zhou XR, et al. Prediction of recurrence and prognosis in patients with hepatocellular carcinoma after resection by use of CLIP score. World J Gastroenterol 2002;8:237-42. [PubMed]
- Cho YK, Chung JW, Kim JK, et al. Comparison of 7 staging systems for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancer 2008;112:352-61. [PubMed]
- Huitzil-Melendez FD, Capanu M, O’Reilly EM, et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol 2010;28:2889-95. [PubMed]
- Collette S, Bonnetain F, Paoletti X, et al. Prognosis of advanced hepatocellular carcinoma: comparison of three staging systems in two French clinical trials. Ann Oncol 2008;19:1117-26. [PubMed]
- Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol 2003;38:207-15. [PubMed]
- Kudo M, Chung H, Haji S, et al. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology 2004;40:1396-405. [PubMed]
- Kondo K, Chijiiwa K, Nagano M, et al. Comparison of seven prognostic staging systems in patients who undergo hepatectomy for hepatocellular carcinoma. Hepatogastroenterology 2007;54:1534-8. [PubMed]
- Ikai I, Takayasu K, Omata M, et al. A modified Japan Integrated Stage score for prognostic assessment in patients with hepatocellular carcinoma. J Gastroenterol 2006;41:884-92. [PubMed]
- Kitai S, Kudo M, Minami Y, et al. A new prognostic staging system for hepatocellular carcinoma: value of the biomarker combined Japan integrated staging score. Intervirology 2008;51 Suppl 1:86-94. [PubMed]
- Yen YH, Changchien CS, Wang JH, et al. A modified TNM-based Japan Integrated Score combined with AFP level may serve as a better staging system for early-stage predominant hepatocellular carcinoma patients. Dig Liver Dis 2009;41:431-41. [PubMed]
- Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer 2002;94:1760-9. [PubMed]
- Chan SL, Mo FK, Johnson PJ, et al. Prospective validation of the Chinese University Prognostic Index and comparison with other staging systems for hepatocellular carcinoma in an Asian population. J Gastroenterol Hepatol 2011;26:340-7. [PubMed]
- Shao YY, Lu LC, Lin ZZ, et al. Prognosis of advanced hepatocellular carcinoma patients enrolled in clinical trials can be classified by current staging systems. Br J Cancer 2012;107:1672-7. [PubMed]
- Chevret S, Trinchet JC, Mathieu D, et al. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol 1999;31:133-41. [PubMed]
- Cammà C, Di Marco V, Cabibbo G, et al. Survival of patients with hepatocellular carcinoma in cirrhosis: a comparison of BCLC, CLIP and GRETCH staging systems. Aliment Pharmacol Ther 2008;28:62-75. [PubMed]
- Huang YH, Wu JC, Chen CH, et al. Comparison of recurrence after hepatic resection in patients with hepatitis B vs. hepatitis C-related small hepatocellular carcinoma in hepatitis B virus endemic area. Liver Int 2005;25:236-41. [PubMed]
- Benzoni E, Lorenzin D, Favero A, et al. Liver resection for hepatocellular carcinoma: a multivariate analysis of factors associated with improved prognosis. The role of clinical, pathological and surgical related factors. Tumori 2007;93:264-8. [PubMed]
- Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821-9. [PubMed]
- Reddy SK, Steel JL, Chen HW, et al. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology 2012;55:1809-19. [PubMed]
- Hoshida Y, Toffanin S, Lachenmayer A, et al. Molecular classification and novel targets in hepatocellular carcinoma: recent advancements. Semin Liver Dis 2010;30:35-51. [PubMed]
- Li S, Mao M. Next generation sequencing reveals genetic landscape of hepatocellular carcinomas. Cancer Lett 2013;340:247-53.
- Boyault S, Rickman DS, de Reyniès A, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 2007;45:42-52. [PubMed]
- Villanueva A, Hoshida Y, Battiston C, et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology 2011;140:1501-12.e2.
- Nault JC, De Reyniès A, Villanueva A, et al. A hepatocellular carcinoma 5-gene score associated with survival of patients after liver resection. Gastroenterology 2013;145:176-87. [PubMed]
- Kaseb AO, Morris JS, Hassan MM, et al. Clinical and prognostic implications of plasma insulin-like growth factor-1 and vascular endothelial growth factor in patients with hepatocellular carcinoma. J Clin Oncol 2011;29:3892-9. [PubMed]



